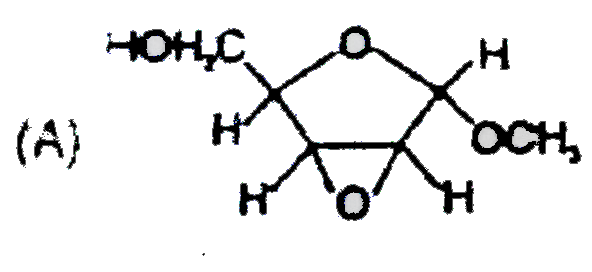
A
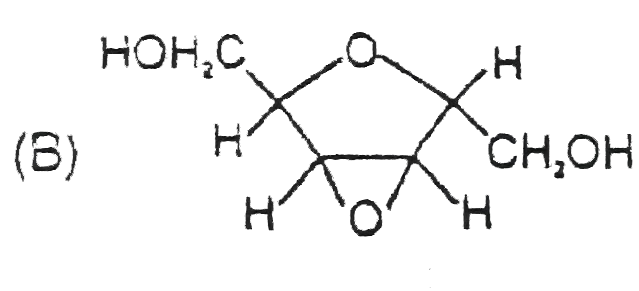
B
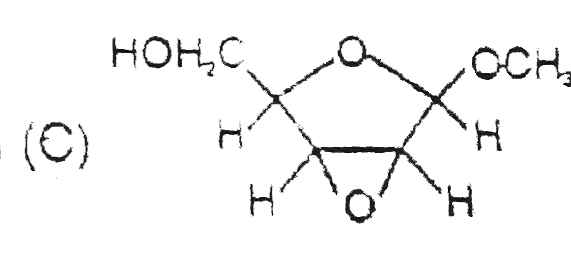
C
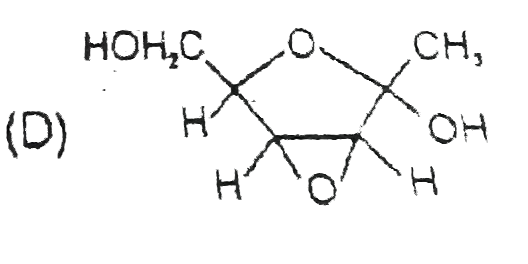
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Which compound does not have zero dipole moment ?
Text Solution
|
- Which reaction takes place during respiration?
Text Solution
|
- Which of the following isomer has lowest boiling point ?
Text Solution
|
- Decreasing order of boiling point of I to IV follow {:("Methylformat...
Text Solution
|
- How many grams of CO2 can be prepared from 150 grams of calcium carbon...
Text Solution
|
- Decreasing order of melting point of compound I-IV follows:
Text Solution
|
- In which case first has higher solubility than second ? (I)Phenol, B...
Text Solution
|
- Give equations for the following: Preparation of CO2 from CaCo3
Text Solution
|
- Give equations for the Formation of acetylene.
Text Solution
|
- PhCOOH, PhCH3 can be separated by
Text Solution
|
- Correct order of boiling order is : {:(CH3CH2-I,CH3CH2-Br,CH3CH2-Cl,...
Text Solution
|
- The correct boiling point order is :
Text Solution
|
- Which compound has highest melting point ?
Text Solution
|
- Which of the following compounds form salt with NaHCO3?
Text Solution
|
- SiO2 is reacted with sodium carbonate. What is the gas liberated?
Text Solution
|
- Which compound does not give positive test in Lassaigne's test for nit...
Text Solution
|
- Which of the following has no net dipole moment ?
Text Solution
|
- In which case second has lower boiling point than first ?
Text Solution
|
- In which case first has lower melting point than second ?
Text Solution
|
- Give equations when sodium carbonate is added to water.
Text Solution
|