A
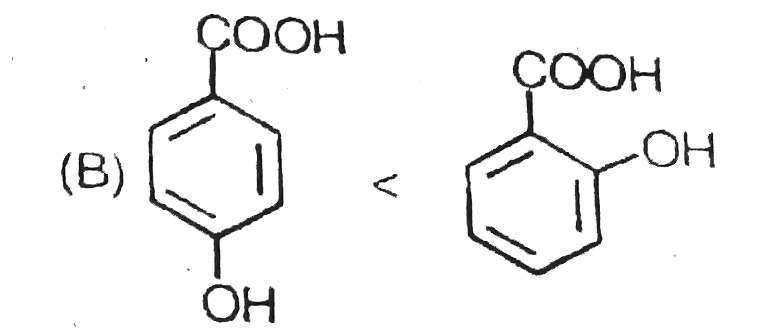
B
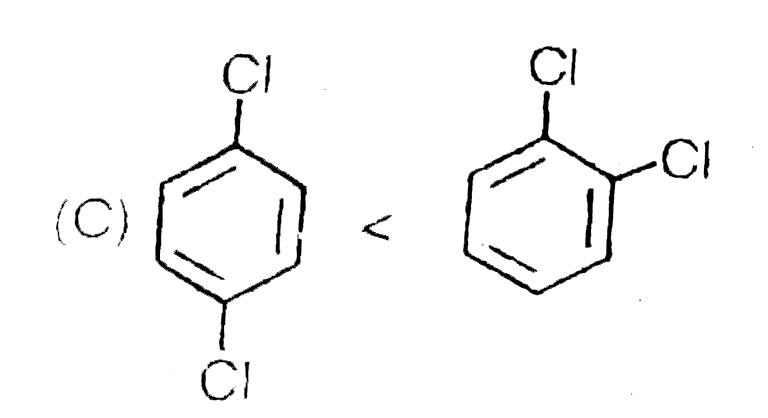
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- In which case first has lower melting point than second ?
Text Solution
|
- Give equations when sodium carbonate is added to water.
Text Solution
|
- Correct water solubility order amongst the following pair is/are :
Text Solution
|
- Which of the following compounds have some solubility in water ?
Text Solution
|
- Which is/are the correct method for separating a mixture of benzoic ac...
Text Solution
|
- does Acetic acid produce CO2 on reaction with NaHCO3
Text Solution
|
- Statement-1:Cis form will always have more boiling point than transfor...
Text Solution
|
- Statement-1:p-Hydroxybenzoic acid has a lower boiling point that o-Hyd...
Text Solution
|
- Statement-1:A mixture of p-Methylbenzoic acid and picric acid is separ...
Text Solution
|
- State true or false : Carbon monoxide is a poisonous gas.
Text Solution
|
- Give one method for industrial preparation CO
Text Solution
|
- Give one method for industrial preparation CO2
Text Solution
|
- Compare the properties of two isomeric products x and y formed in the ...
Text Solution
|
- What are the properties of CO2?
Text Solution
|
- How many compounds have zero dipole-moment ? (a)CH3Cl , (b)CHCl5 , (...
Text Solution
|
- Amongest the following, the total number of compound soluble in aqueou...
Text Solution
|
- Carbon monoxide is posionous because it ?
Text Solution
|
- Different reagents used for the identification of different functional...
Text Solution
|
- Different reagents used for the identification of different functional...
Text Solution
|
- Silicon is the most abundant element on earth(T/F)
Text Solution
|