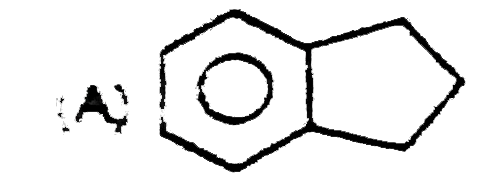
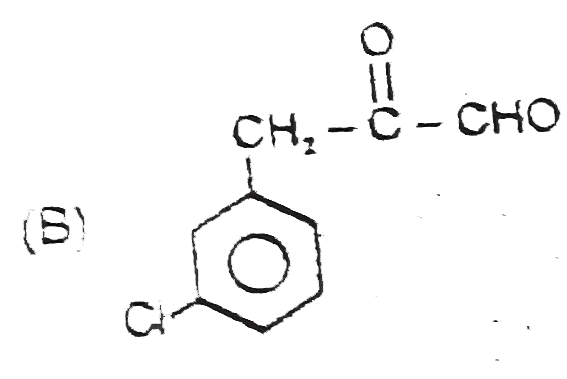
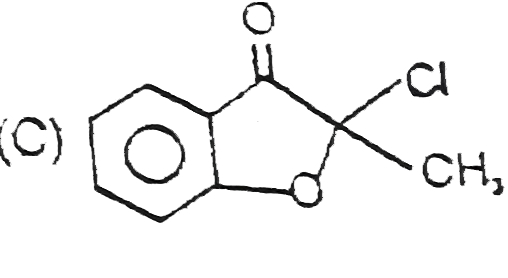
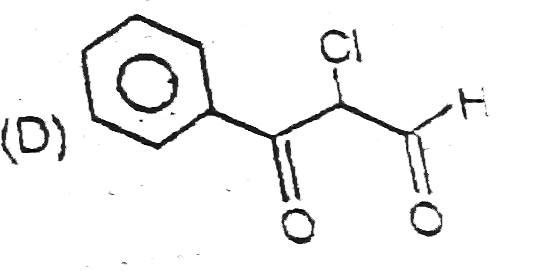
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Calculate total number of possible isomers (structrural and stereoisom...
Text Solution
|
- The compound X (C9H7CIO2) is optically active.It mainly exists in enol...
Text Solution
|
- Which of the following compound give methane on treatment with CH3MgI
Text Solution
|
- The product ( C) of the following reactions is
Text Solution
|
- Phenyl acetate underset((ii)H^(o+))overset((i)CH3MgBr("excess"))to'P' ...
Text Solution
|
- No of products and number of fractions are respectively :
Text Solution
|
Text Solution
|
- Complete the following reaction
Text Solution
|
- Among the following which statement is/are correct. (A)CH3-oversetov...
Text Solution
|
- Which statement about formaldehyde is correct
Text Solution
|
- CH2=CH-CH2OHundersetIoverset(PhH//HF)toAunderset(II)overset(H2//Pt)toB...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Aniline reacts with which of the following to form Schiff's Base ?
Text Solution
|
- Partial reduction of Cyanides with SnCl2 / HCl gives X which on hydrol...
Text Solution
|
- The correct compounds statement is /are :
Text Solution
|
- Both the C(beta)-H and Calpha-X bonds are breaking in the transition s...
Text Solution
|
- Calculate the weight of lime (CaO) obtained by heating 290kg of 95% pu...
Text Solution
|
- The density (in g ml^(−1) ) of a 3.60M sulphuric acid solution having...
Text Solution
|
- The density (in g ml^(−1) ) of a 3.60M sulphuric acid solution having...
Text Solution
|
- Observe the following reaction sequence carefully.Find the total numbe...
Text Solution
|