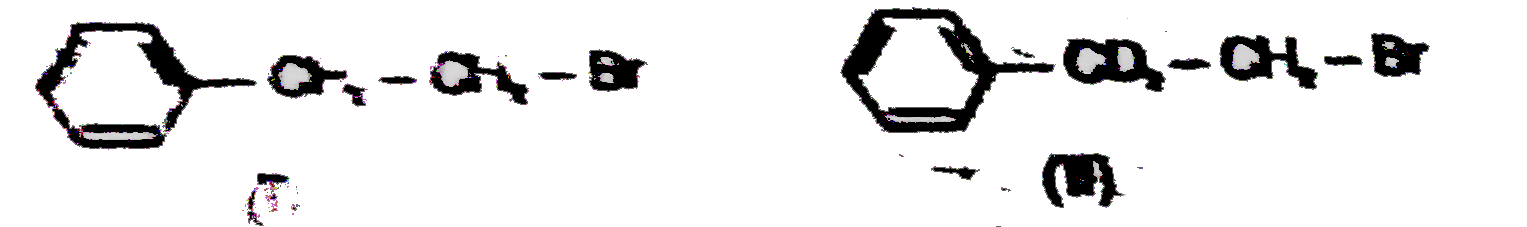A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Partial reduction of Cyanides with SnCl2 / HCl gives X which on hydrol...
Text Solution
|
- The correct compounds statement is /are :
Text Solution
|
- Both the C(beta)-H and Calpha-X bonds are breaking in the transition s...
Text Solution
|
- Calculate the weight of lime (CaO) obtained by heating 290kg of 95% pu...
Text Solution
|
- The density (in g ml^(−1) ) of a 3.60M sulphuric acid solution having...
Text Solution
|
- The density (in g ml^(−1) ) of a 3.60M sulphuric acid solution having...
Text Solution
|
- Observe the following reaction sequence carefully.Find the total numbe...
Text Solution
|
- Monocarbonyl Compound X underset("Clemmensen Reduction")overset(Zn-Hg/...
Text Solution
|
- In the given structure at how many atoms the positive charge will be d...
Text Solution
|
- The total number of isomeric products (including stereomers ) formed a...
Text Solution
|
- How many alkenes are possible in the given below reaction ?
Text Solution
|
- How many moles of acetic anhydride (Ac2O) is required to react complet...
Text Solution
|
- At a certain temperature the following equilibrium is established CO(g...
Text Solution
|
- A monatomic ideal gas undergoes a process in which the ratio of P to V...
Text Solution
|
- BF3 & C Cl4 on reaction with water produce & respectively:
Text Solution
|
- For the following flow diagram : Which of the following option de...
Text Solution
|
- For the given reaction the correct reactivity order of H-atoms is -
Text Solution
|
- x g of H2O2 requires 100mL of M//5 KMnO4 in a titration in a solution ...
Text Solution
|
- The solubility of a sparingly soluble salt AxBy in water at 25^@C=1.5x...
Text Solution
|
- Which of the following property of alkali metals/alkali metal salt inc...
Text Solution
|