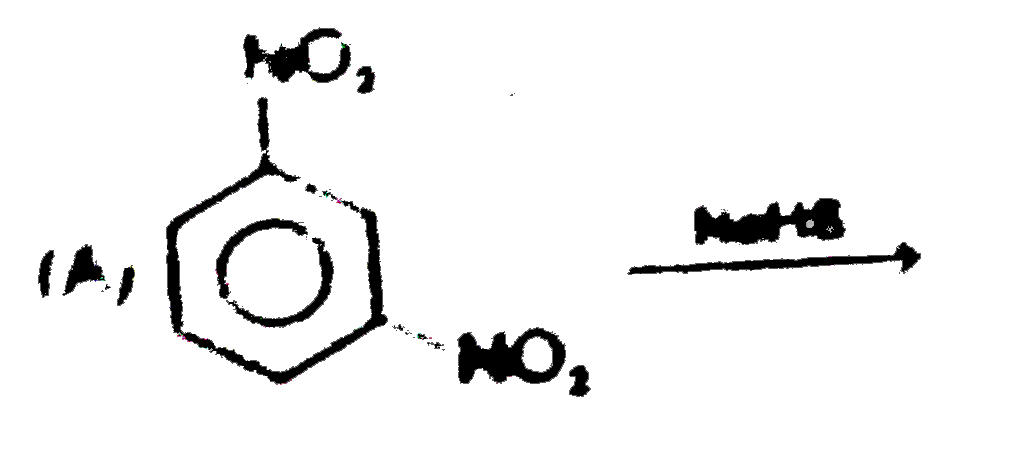
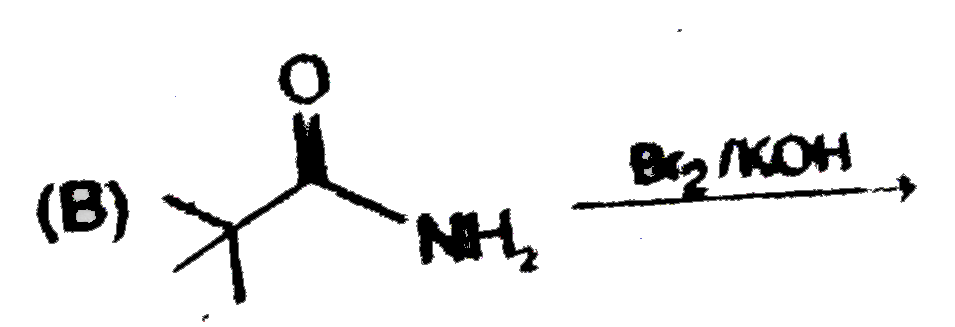
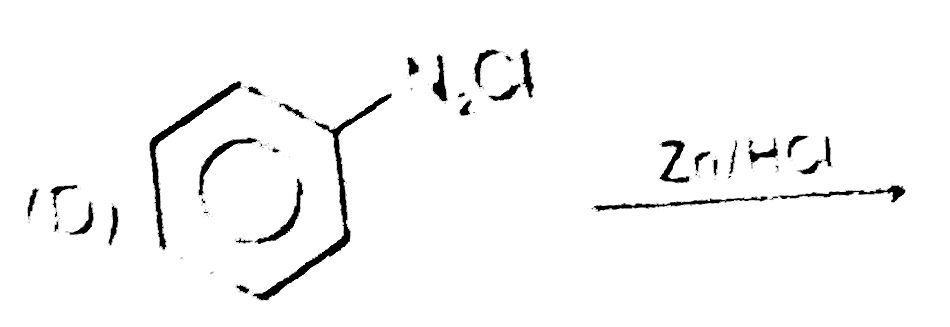A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-RANK BOOSTER-All Questions
- Which of the following statement is correct for the thiosulphate test ...
Text Solution
|
- Which of the following are correctly matched ?
Text Solution
|
- Primary (1^@) amine group is formed in :
Text Solution
|
- The main application of osmotic pressure measurement is in the determi...
Text Solution
|
- Give any two types of molar masses for polymers
Text Solution
|
- Answer the questions on the basis of following observations The c...
Text Solution
|
- Answer the questions on the basis of following observations The c...
Text Solution
|
- EANof[Fe(eta^(5)-C(5)H(5))(CO)(2)CI]:
Text Solution
|
- The brown ring complex compound is formulated as [Fe(H(2)O)(5)(NO)]SO(...
Text Solution
|
- 1g of a monobasic acid dissolved in 200g of water lowers the freezing ...
Text Solution
|
- underset((ii). (H^(+))/(H(2)O))overset((i). CH(3)MgBr (3.0 "equivalent...
Text Solution
|
- What is Ninhydrin test used for ?
Text Solution
|
- Which of the following statements is correct with respect to the metal...
Text Solution
|
- The following flow diagram represents the extraction of magnesium from...
Text Solution
|
- Write the order of thermal stability of the hydrides of Group 16 elem...
Text Solution
|
- Given that the normal energy of the reactant and produce are 40J and 2...
Text Solution
|
- An aqueous solution of NaCI freezes at -0.186^(@)C. Given that K(b(H(2...
Text Solution
|
- The average charge on each O atom and average bond order of S-O bond i...
Text Solution
|
- Complete the following reaction
Text Solution
|
Text Solution
|