A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise MISCELLANEOUS SOLVED PROBLEMS|7 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise Board Level Exercise|31 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise Advanced Level Problems|105 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise APSP PART-3|22 VideosQUALITATIVE ANALYSIS
RESONANCE ENGLISH|Exercise INORGANIC CHMISTRY(Qualitative analysis)|35 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-PERIODIC TABLE & PERIODICITY-Example
- An element X with Z = 112 has been recently discovered. What is the el...
Text Solution
|
- Atomic radius of Li is 1.23 Å and ionic radius of Li^(+) is 0.76 Å. C...
Text Solution
|
- Select from each group the species which has the smallest radius start...
Text Solution
|
- Mg^(2+) is smaller than O^(2-) in size, throgh both have same electron...
Text Solution
|
- From each set, choose the atom which has the largest ionization enthal...
Text Solution
|
- First and second ionisation energies of magnesium are 7.646 eV and 15....
Text Solution
|
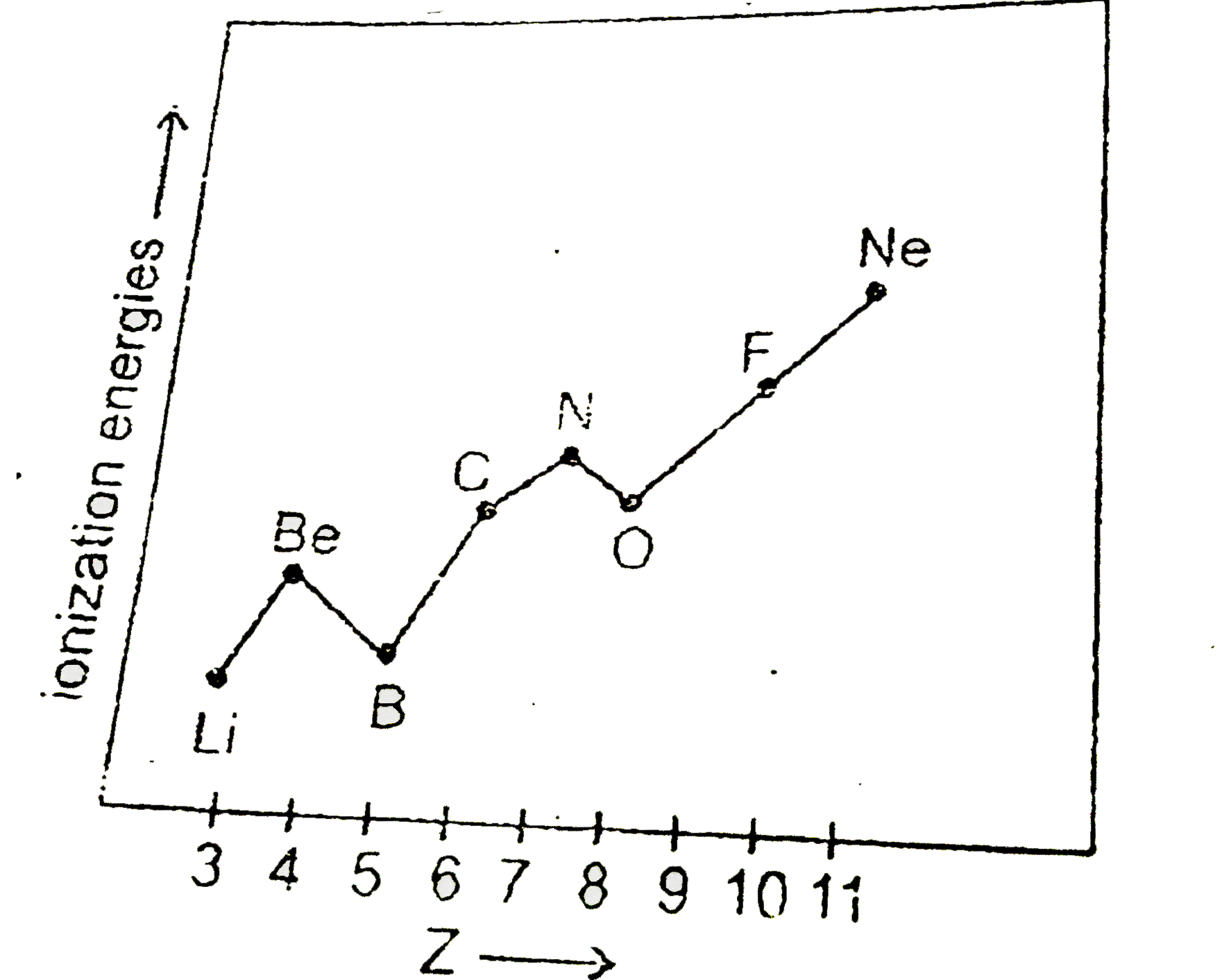
- Following graph shows variation of ionization energies with atomic num...
Text Solution
|
- Consider the following ionization stesps: M(g) ot M^(+)(g)+e^(-)," ...
Text Solution
|
- Consider the elements N, P, O and S and arrange them in order of incre...
Text Solution
|
- Why do halogens have high electron gain enthalpies (i.e. -Delta(eg)H^(...
Text Solution
|
- Which will have the maximum value of electron affinity O^(x),O^(y),O^(...
Text Solution
|
- The amount of energy when million atoms of iodine are completely conve...
Text Solution
|
- Account for the large decrease in electron affinity between Li and Be ...
Text Solution
|