Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise Exercise-2|1 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise Exercise-3|1 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise Exercise-1|1 VideosP-BLOCK ELEMENTS (HALOGEN & NOBLE GASES)
RESONANCE ENGLISH|Exercise APSP PART-3|22 VideosQUALITATIVE ANALYSIS
RESONANCE ENGLISH|Exercise INORGANIC CHMISTRY(Qualitative analysis)|35 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-PERIODIC TABLE & PERIODICITY-Exercise
- Report atomic number of the element having largest size among the foll...
Text Solution
|
- Find the total number of elements which have higher ionisation energy ...
Text Solution
|
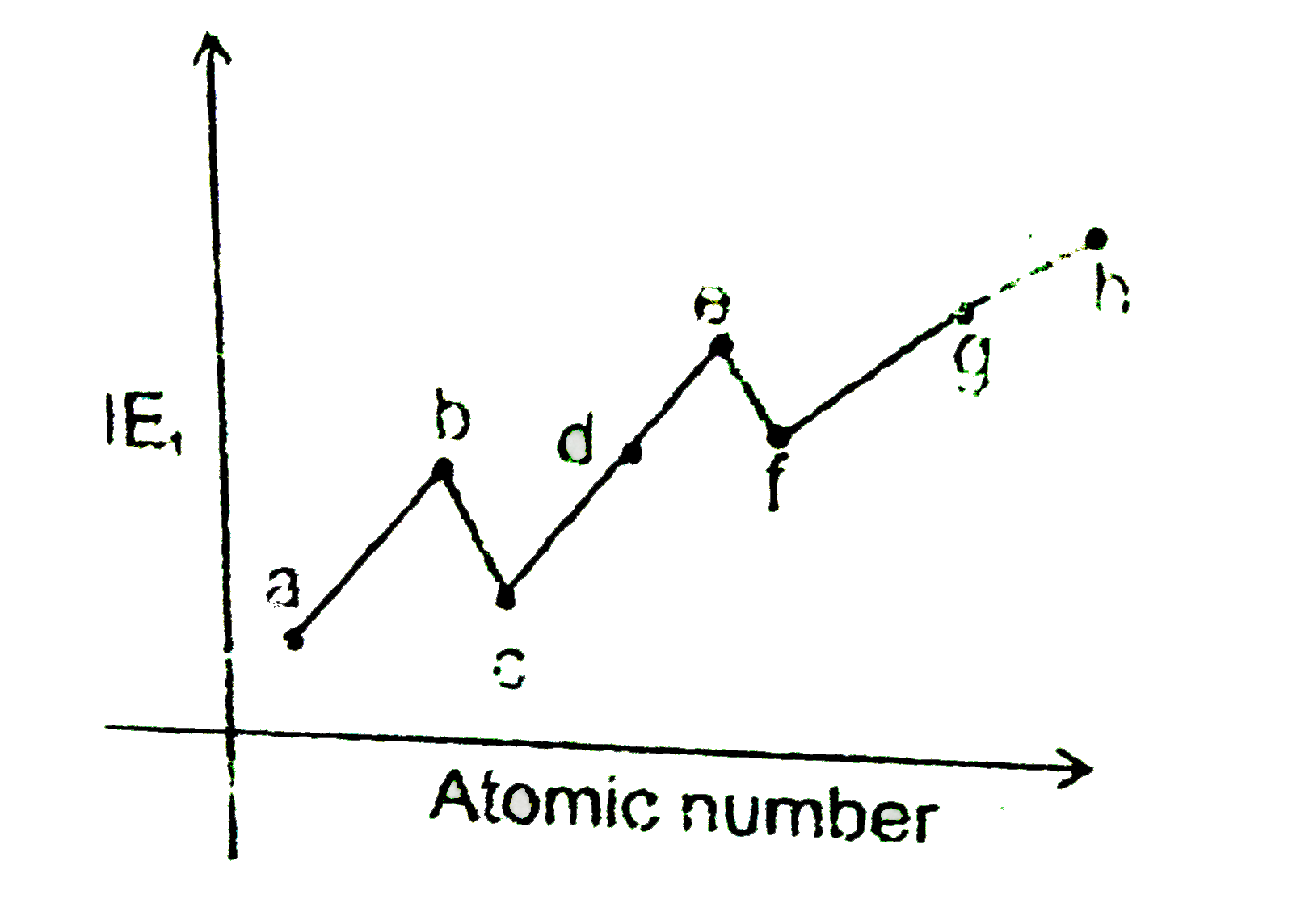
- Where a, b,c, d, e,f,g, h ar 3^(rd) period elements. If difference bet...
Text Solution
|
- Value of IE(1), IE(2), IE(3) of an element are 9.3, 18.2 and 553.8 eV....
Text Solution
|
- Which one of the following elements has lowest ionisation energy: H,...
Text Solution
|
- {:(A^(-) (g) rarr A^(2+)(g),,DeltaH = 1100KJ//mol),(A(g)rarrA^(2+)(g),...
Text Solution
|
- The electron affinity of a hypothetical element 'A' is 3eV per atom. H...
Text Solution
|
- What is atomic number of element which have maximum electron affinity ...
Text Solution
|
- How many of the following are more electronegativity than Boron. H, Li...
Text Solution
|
- The group in the modern periode table, in which all the elements do no...
Text Solution
|
- Element corresponding to which of these//this atomic number belongs to...
Text Solution
|
- Which of the following have greater Z(eff) than Zn:
Text Solution
|
- Which of the following is/are correct regarding oxidation state of ele...
Text Solution
|
- Which of the following elements have +3 as most popular oxidation stat...
Text Solution
|
- Which of the following compounds are found to exist (General formula o...
Text Solution
|
- Which of the following show non-zero multiple oxidation state?
Text Solution
|
- Which of the following pairs of elements show similar set of oxidation...
Text Solution
|
- Which of the following elements have their lower oxidation state as mo...
Text Solution
|
- Which is /are the correct order/s of atomic radius?
Text Solution
|
- Which is/are the correct order/s of atomic radius?
Text Solution
|