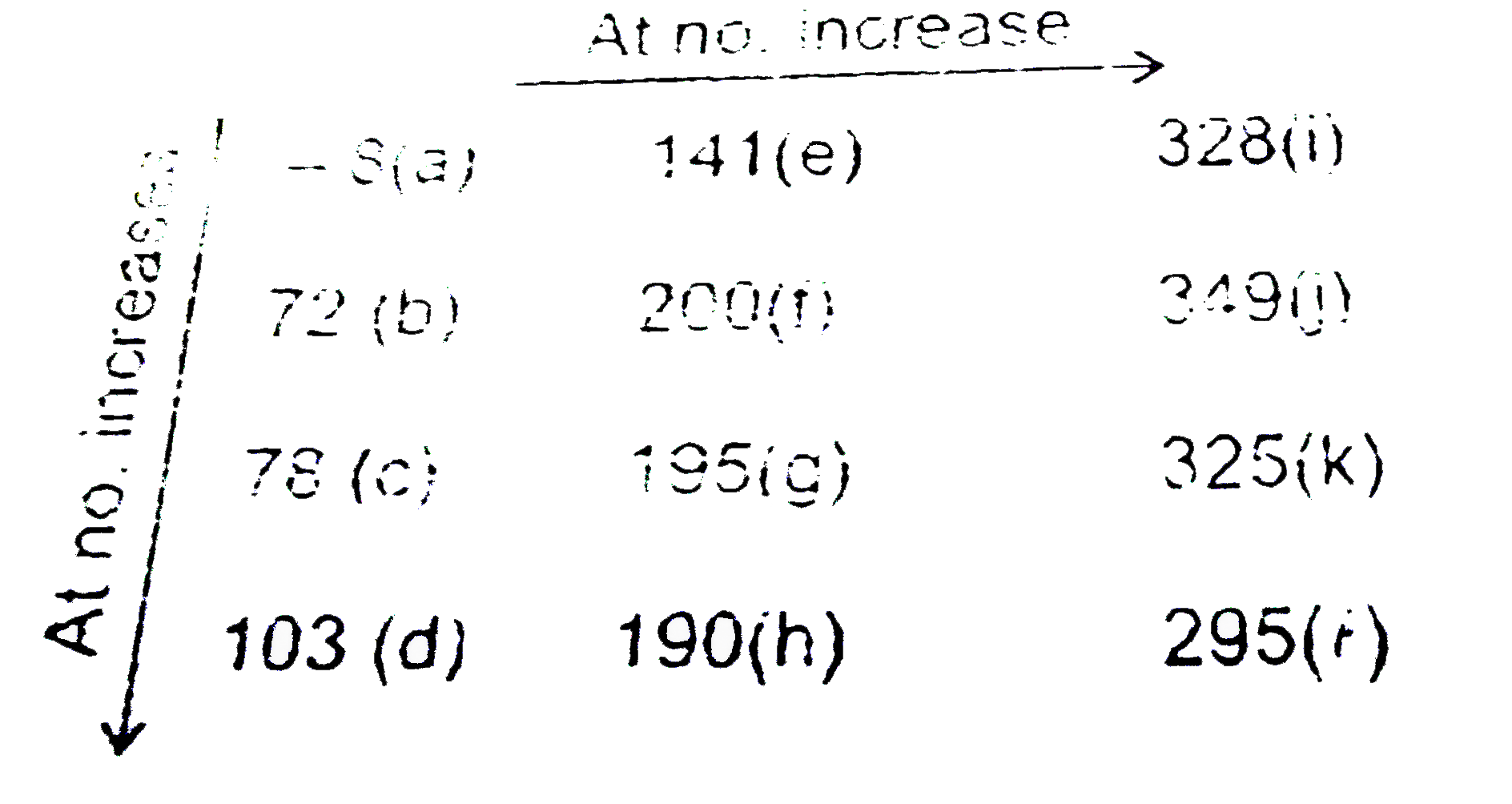
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-PERIODIC TABLE & PERIODICITY-Advanced Level Problems
- EA(1) value of some group of p-Block elements are given: a, b, c…...
Text Solution
|
- EA(1) value of some group of p-Block elements ae given: a, b, c……...
Text Solution
|
- EA(1) value of some group of p-Block elements ae given: a, b, c……...
Text Solution
|
- The element whose electronic configuration is 1s^(2), 2s^(2)2p^(6), 3s...
Text Solution
|
- Oxygen shows +2 oxidation state is
Text Solution
|
- Which Group IIIA elements is expected to have physical and chemical pr...
Text Solution
|
- Which of the followig ions will show highest magnetic moment (Z values...
Text Solution
|
- Find the oxidation number of: Cr in K(2)Cr(2)O(7)
Text Solution
|
- Which of the following is the smallest in size ?
Text Solution
|
- Oxidation Number of Mn in [MnO(4)]^(-) is:
Text Solution
|
- The reduction potentials of Zn, Cu, Fe and Ag are in the increasing or...
Text Solution
|
- The formation of anion from a neutral atom X is favoured by:
Text Solution
|
- An isotone of .(32)^(76)Ge is- (a) .(32)^(77)Ge (b).(33)^(77)As (c).(...
Text Solution
|
- Which element of 3rd row has biggest atomic size?
Text Solution
|
- Which group of periodic table have large negative energy of activation...
Text Solution
|
- The species which has its fifth ionization potential equal to 340 V is...
Text Solution
|
- The atom of an element X contains 27 electron X is expected to be
Text Solution
|
- The group in the periodic table that contains the elements in all the ...
Text Solution
|
- The ion having a noble gas electronic configuration is
Text Solution
|
- Element with Z = 83 belongs to which block?
Text Solution
|