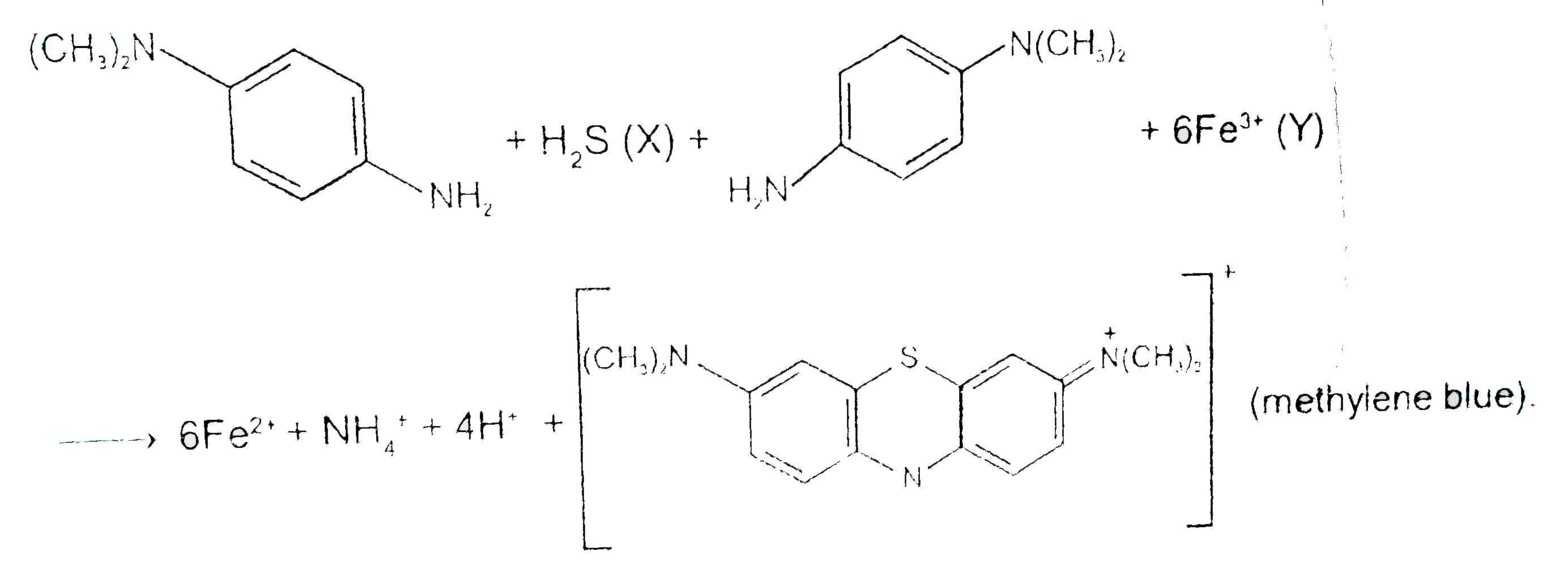A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-QUALITATIVE ANALYSIS PART 1-EXERCISE 3
- A metal nitrate reacts with KI to give a black precipitate which on ad...
Text Solution
|
- A solution when diluted with H(2)O And boiled gives a white precipita...
Text Solution
|
- In blue solution of copper sulphate excess of KCN is added then solut...
Text Solution
|
- MgSO(4) +NH(4)OH + Na(2)HPO(4) to white crystalline precipitate.The fo...
Text Solution
|
- A solution of a metal ion when treated with KI gives a red precipitate...
Text Solution
|
- A solution of colourless salt on boiling with excess NaOH produces a n...
Text Solution
|
- p-Amino-N,N-dimethylaniline is added to a strongly acidic solution of ...
Text Solution
|
- p-Amino-N,N-dimethylaniline is added to a strongly acidic solution of ...
Text Solution
|
- p-Amino-N,N-dimethylaniline is added to a strongly acidic solution of ...
Text Solution
|
- When a metal rod M is dipped into an aqueous colourless concetrated so...
Text Solution
|
- When a metal rod M is dipped into an aqueous colourless concetrated so...
Text Solution
|
- When a metal rod M is dipped into an aqueous colourless concetrated so...
Text Solution
|
- In an acidified aqueous solution of Mn^(2+), Ni^(2+), Cu^(2+) and hg^(...
Text Solution
|
- The equilibrium 2 Cu^(I)hArr Cu+Cu^(II) In aqueous medium at 25^@C ...
Text Solution
|
- For the given aqueous reaction which of the statement(s) is (are) true...
Text Solution
|
- Concentrated HNO3, upon long standing, turns yellow-brown due to the f...
Text Solution
|
- Upon treatment with ammonical H(2)S solution, the metal ion precipitat...
Text Solution
|
- An aqueous solution of a mixture of two inorganic salts when treated w...
Text Solution
|
- An aqueous solution of a mixture of two inorganic salts when treated w...
Text Solution
|
- Which one of the following statement is correct?
Text Solution
|