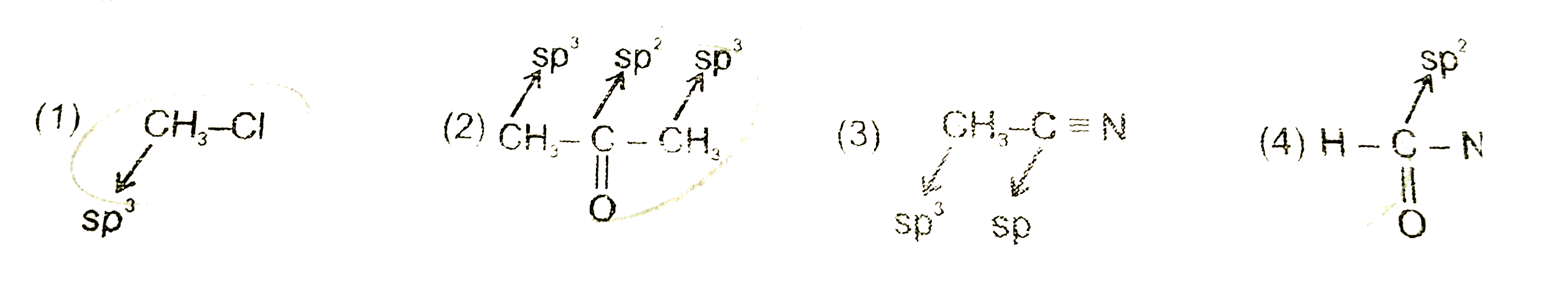A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE ENGLISH|Exercise Exercise-1 Part-1 Section(A)|10 VideosIUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE ENGLISH|Exercise Exercise-1 Part-1 Section(B)|8 VideosIUPAC NOMENCLATURE & STRUCTURAL ISOMERISM
RESONANCE ENGLISH|Exercise Advanced Level Problems Part-5|2 VideosIONIC EQUILIBRIUM
RESONANCE ENGLISH|Exercise partIII one or more than one options correct type|10 VideosMETALLURGY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Alkyl Halide, Alcohol,Phenol,Ether)|17 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-IUPAC NOMENCLATURE & STRUCTURAL ISOMERISM -Bord Level Exercise
- Write the structure of following IUPAC name: (i)1,1-Dibromo-3-ethyl-...
Text Solution
|
- Write the structure of following IUPAC name: (i)2-Methylpentane-2,4-...
Text Solution
|
- Write the correct IUPAC name of the following: (i)H(3)C-undersetunde...
Text Solution
|
- Write the structure of following IUPAC name: (i)3-Etylnhexa-1,4-dien...
Text Solution
|
- Write the correct IUPAC name of the following: (i) H(3)C-undersetund...
Text Solution
|
- Write the correct IUPAC name of the following: (i)
Text Solution
|
- Write the structure of following IUPAC name: (i)2-Methoxybutan-1-ol ...
Text Solution
|
- Write the correct IUPAC name of the following:
Text Solution
|
- What is isomerism? Give an example.
Text Solution
|
- What is positional isomerism? Give an example.
Text Solution
|
- What is metamerism
Text Solution
|
- Identify the molecular weight of the compound 'X' containing carbon an...
Text Solution
|
- Write the ring chain functional isomer of compound But-2-ene are?
Text Solution
|
- In the following reaction, A+B+Q⇌C+D the temperature is increased then...
Text Solution
|
- Identify the relationship between the give compounds.
Text Solution
|
- Write down the IUPAC name of the following compound: (a)Methyl benzo...
Text Solution
|
- What is the type of hybridisation of each carbon in the following comp...
Text Solution
|
- Which of the following represents the correct IUPAC name for the compo...
Text Solution
|
- Write common & IUPAC name of the following structure:
Text Solution
|
- Identify the relationship between the give compounds.
Text Solution
|