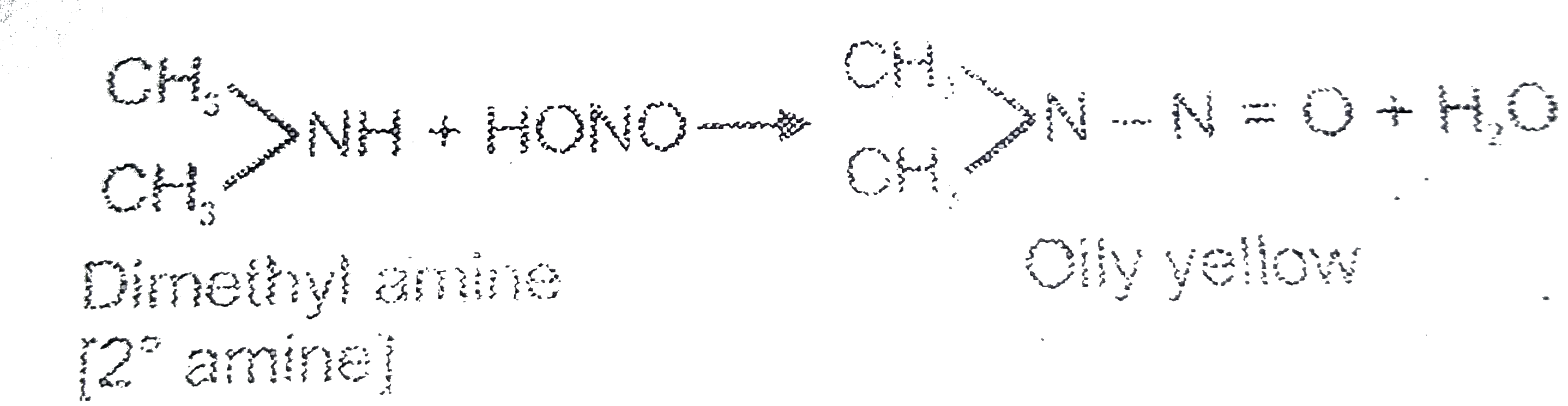
Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Exercise-1 (Part-I)|23 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Exercise-1 (Part-II)|37 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))|23 VideosSURFACE CHEMISTRY
RESONANCE ENGLISH|Exercise Section - 5|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-STRUCTURAL IDENTIFICATION-Advanced level Problems (Part-III)
- An organic compound A having molecular formula C2 H7 N on treatment ...
Text Solution
|
- What simple laboratory test could be performed to distinguish between ...
Text Solution
|
- A compound (A) has molecular formula C(5)H(9)Cl .It does not re...
Text Solution
|
- Which of the following tests could be performed to distinguish between...
Text Solution
|
- What is Pyrolysis ? Explain the pyrolysis of Dodecane ?
Text Solution
|
- Lucas reagent consists of
Text Solution
|
- The percentage of nitrogen in a compound is determined by
Text Solution
|
- The percentage of nitrogen in a compound is determined by
Text Solution
|
- In the Dumas method for the estimation of nitrogen , 0.0237 grams of a...
Text Solution
|
- Tollen's reagent is
Text Solution
|
- The compound used in the treatment of lead poisoning is :
Text Solution
|
- Fehlings solution is
Text Solution
|
- Which of the following phenols is most soluble in aqueous sodium bicar...
Text Solution
|