A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
STRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Exercise - 2 (Part-I)|10 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Exercise - 2 (Part-II)|8 VideosSTRUCTURAL IDENTIFICATION
RESONANCE ENGLISH|Exercise Exercise-1 (Part-I)|23 VideosSTEREOISOMERISM
RESONANCE ENGLISH|Exercise EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))|23 VideosSURFACE CHEMISTRY
RESONANCE ENGLISH|Exercise Section - 5|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-STRUCTURAL IDENTIFICATION-Exercise-1 (Part-II)
- Which of the following compounds gives red ppt with Cu(2)Cl(2)//NH(4)O...
Text Solution
|
- Identify the hydrocarbon having molecular formula C(5)H(6) which gives...
Text Solution
|
- The group reagent for the test of alcohols is :
Text Solution
|
- The following two compounds I and II can be distinguished by using rea...
Text Solution
|
- Which of the following compound will not react with l(2)//OH^(-).
Text Solution
|
- A compound A gives following reactions. Its structure can be
Text Solution
|
- An organic compound X (C(4)H(8)O(2)) gives positive test with NaOH an...
Text Solution
|
- Which of the following compound will give smell of NH(3) with conc. Na...
Text Solution
|
- Which of the following will not give positive test with CHCl(3)//KOH.
Text Solution
|
- A positive carbylamine test is shown by :
Text Solution
|
- The Hinsberg's method is used for :
Text Solution
|
- The IUPAC name for the following compound is
Text Solution
|
- , Instant turbidity Identyfy X :
Text Solution
|
- Which of the following would produce effervescence with sodium bicarbo...
Text Solution
|
- A compound is heated with zinc dust and ammonium chloride followed by ...
Text Solution
|
- In the Lassaigne's test , one of the organic compound gave red colour ...
Text Solution
|
- Lassaigne's test is used in the qualitative analysis to detect
Text Solution
|
- The compound that does not give a blue colour in Lassaigne’s test is:
Text Solution
|
- Nitrogen containing organic compound when fused with sodium metal form...
Text Solution
|
- The sodium extract of an organic compound on acidified with acetic aci...
Text Solution
|
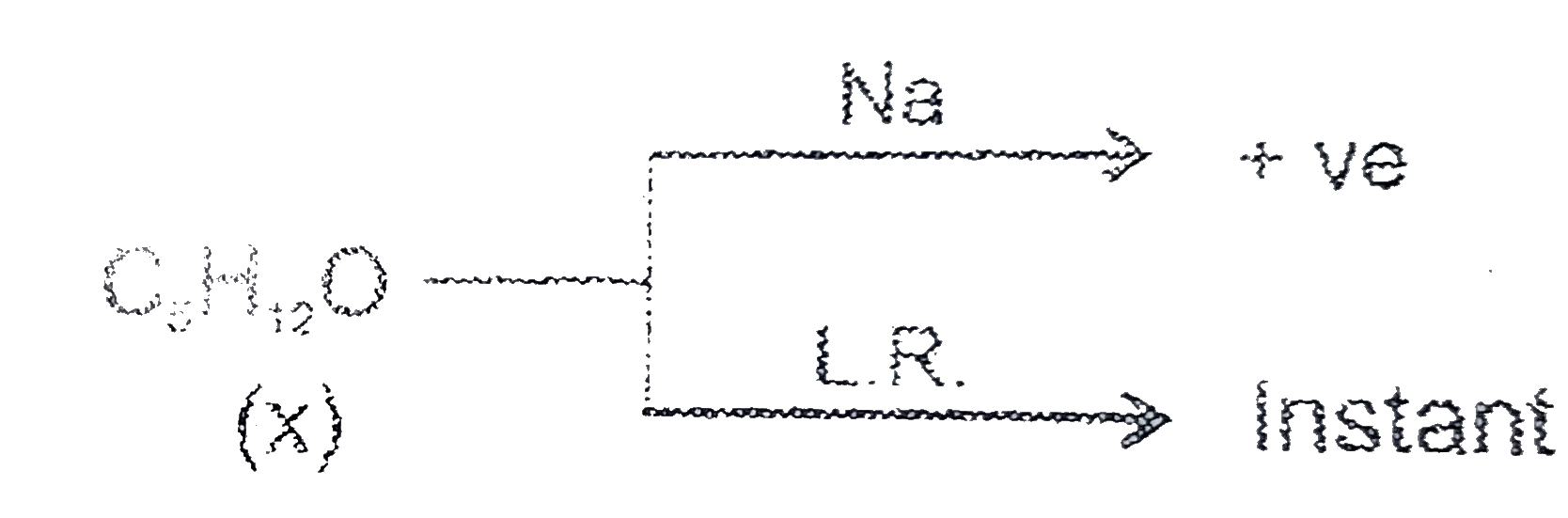 , Instant turbidity
, Instant turbidity