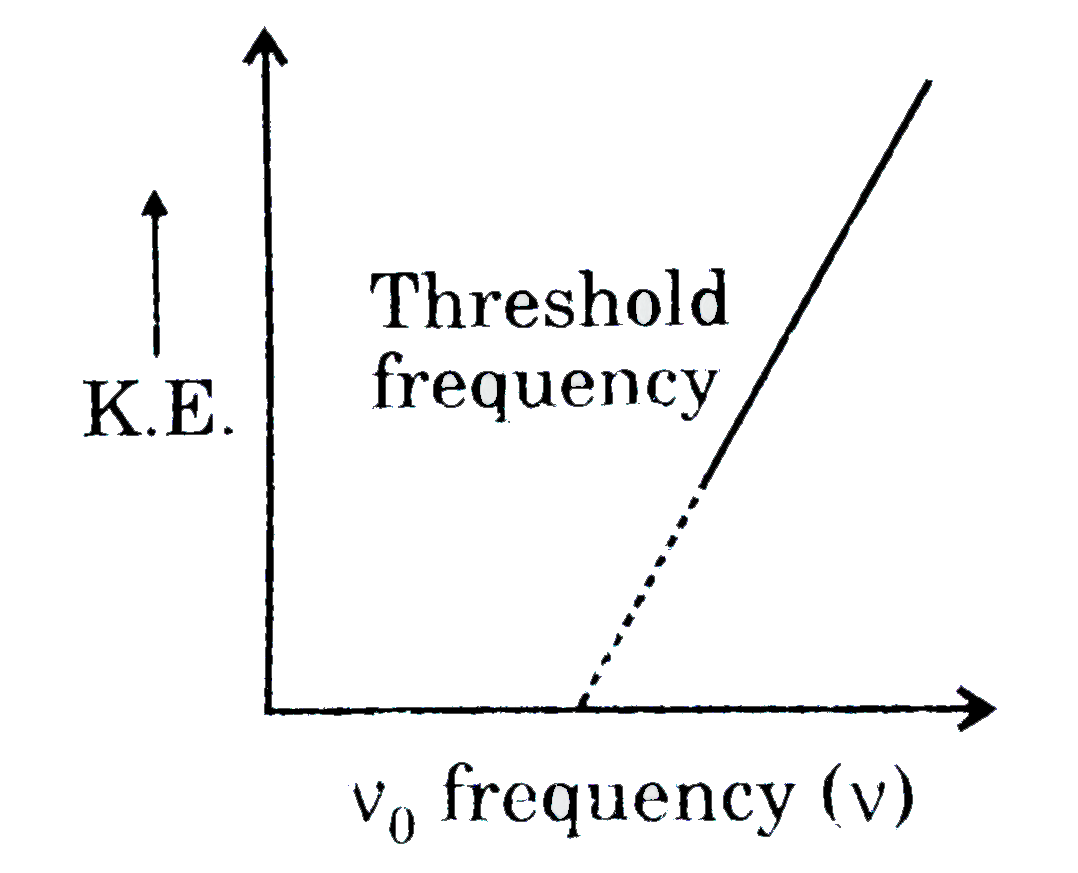
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR CHEMISTRY
RESONANCE ENGLISH|Exercise Exercise-3|15 VideosNUCLEAR CHEMISTRY
RESONANCE ENGLISH|Exercise PART-II|26 VideosNUCLEAR CHEMISTRY
RESONANCE ENGLISH|Exercise PART -III :|8 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise PART -II|23 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-NUCLEAR CHEMISTRY-PART-IV : COMPREHENSION
- A beam of white light is dispersed into its wavelength components of p...
Text Solution
|
- In the photoelectric effect the electrons are emiited instantaneously ...
Text Solution
|
- In the photoelectric effect the eletrons are emiited intantaneously fr...
Text Solution
|
- Last line of bracket series for H-atom has wavelength lambda(1)"Å" an...
Text Solution
|
- (i) First line of a series : It is called line of longest wavelength o...
Text Solution
|
- wave number of the first line of Paschen series in Be^(3+) ion is :
Text Solution
|
- de Broglie proposed dual nature for electron by putting his famous equ...
Text Solution
|
- de Broglie proposed dual nature for electron by putting his famous equ...
Text Solution
|
- de Broglie proposed dual nature for electron by putting his famous equ...
Text Solution
|
- The angular wave function of which orbital with not disturb by the var...
Text Solution
|
- Quantum number area assigned to get complete information of electron...
Text Solution
|
- Quantum number area assigned to get complete inforamtion of electron...
Text Solution
|
- Quantum number area assigned to get complete inforamtion of electron...
Text Solution
|