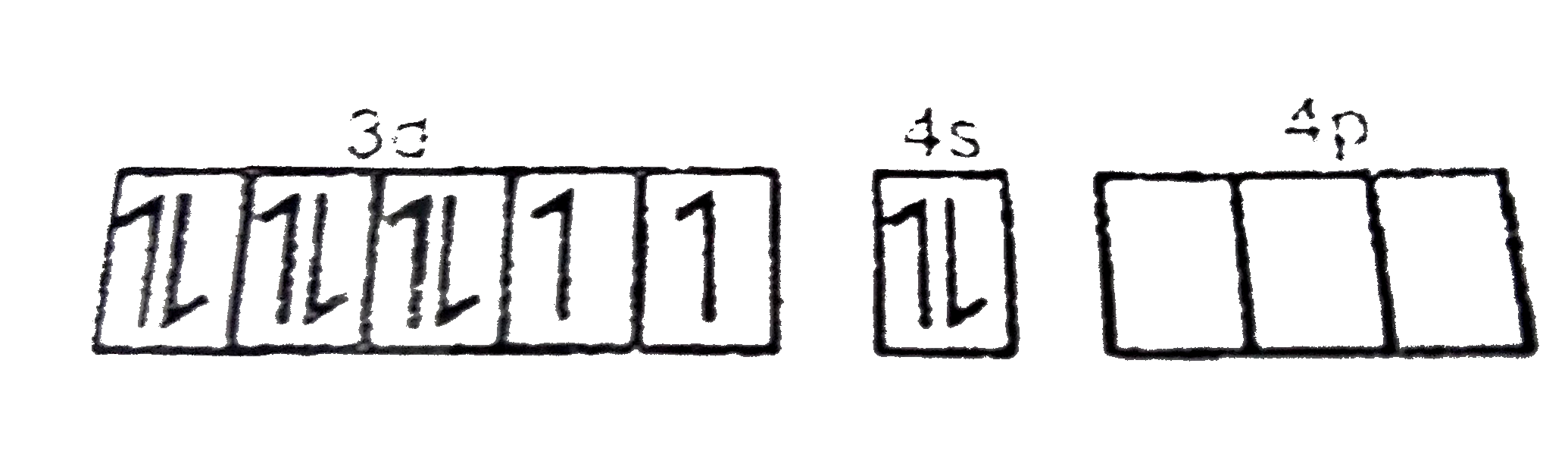A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
NUCLEAR CHEMISTRY
RESONANCE ENGLISH|Exercise ADVANCED LEVEL PROBLEMS|30 VideosNUCLEAR CHEMISTRY
RESONANCE ENGLISH|Exercise PART-II SECTION-1|21 VideosNUCLEAR CHEMISTRY
RESONANCE ENGLISH|Exercise Exercise-3|15 VideosNITROGEN CONTAINING COMPOUNDS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Nitrogen containing Compounds)|30 VideosP BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise PART -II|23 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-NUCLEAR CHEMISTRY-PART-II
- The orbital angular momentum for an electron revolving in an orbit is ...
Text Solution
|
- The wavelength of the radiation emitted, when in a hydrogen atom elec...
Text Solution
|
- which of the following sets of quantum number is correct for an electr...
Text Solution
|
- Consider the ground state Cr atom (Z = 24) The number of electron wi...
Text Solution
|
- Which of the following statements in relation to the hydrogen atom is ...
Text Solution
|
- In a multi-electron atom, which of the following orbitals described by...
Text Solution
|
- Uncertainty in the position of an electron ("mass = "9.1 xx 10^(-31)kg...
Text Solution
|
- According to Bohr's theory, the angular momentum of electron in the fi...
Text Solution
|
- The spin-only magnetic moment [in units of Bohr magneton, (mu(B) of Ni...
Text Solution
|
- Which of the following nuclear reactiosn will generate an isotope ?
Text Solution
|
- The ionisation enthalpy of hydrogen atom is 1.312 xx 10^6" J mol"^(-1)...
Text Solution
|
- Which of the following sets of quantum numbers represents the highest ...
Text Solution
|
- The energy required to break one mole of Cl-Cl bonds in Cl(2) is "242 ...
Text Solution
|
- The ionisation energy of He^(o+) is 19.6 xx 10^(-18) J "atom" ^(-1) ....
Text Solution
|
- A gas absorbs photon of 355 nm and emits at two wavelengths. If one of...
Text Solution
|
- The frequency of light emitted for the transition n =4 to n =2 of He^+...
Text Solution
|
- The electrons identified by quantum numbers n and l :- (a) n=4, l=1 ...
Text Solution
|
- Energy of an electron is given by E=-2.178'10^(-18)J Wavelength of...
Text Solution
|
- The correct set of four quantum numbers of the valence electrons of Ru...
Text Solution
|
- Which of the following is the energy of a possible excited state of hy...
Text Solution
|