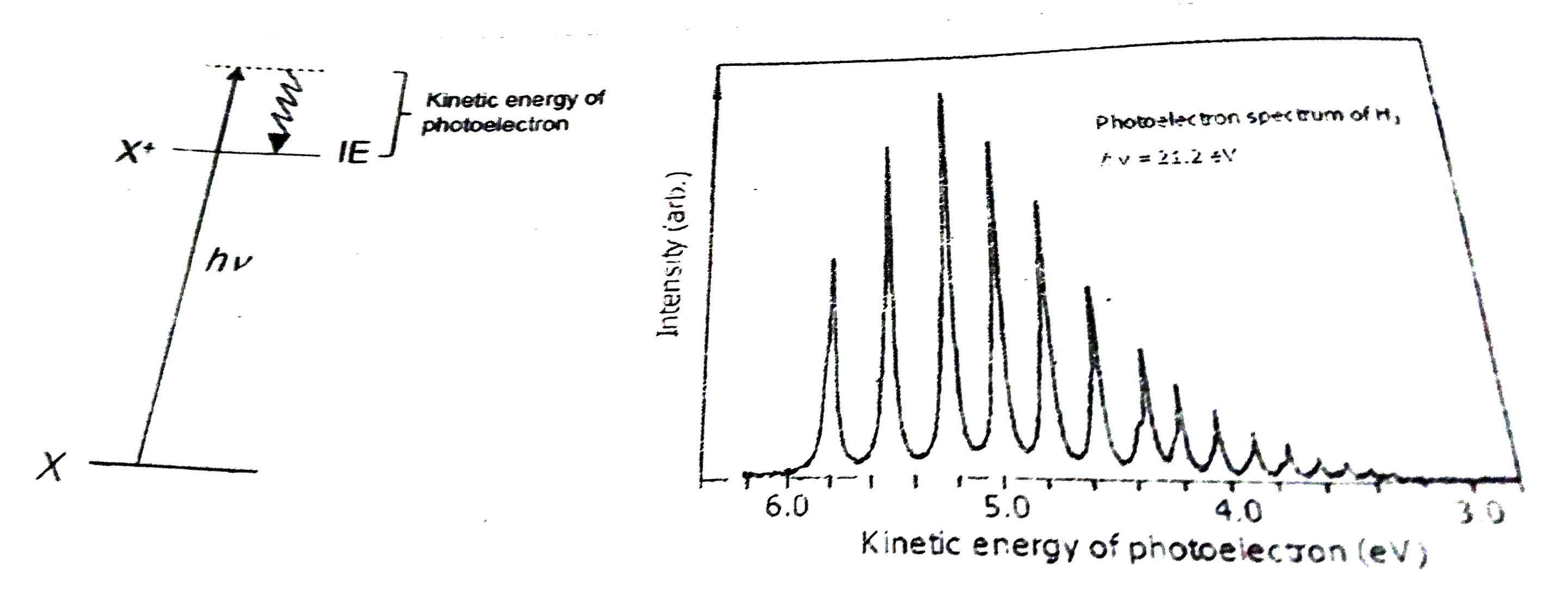
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- When an atom X absorbs radiation with a photon energy than the ionizat...
Text Solution
|
- A photon of energy 15eV collides with H-atom. Due to this collision, H...
Text Solution
|
- When an atom X absorbs radiation with a photon energy than the ionizat...
Text Solution
|
- When an atom X absorbs radiation with a photon energy than the ionizat...
Text Solution
|
- When an atom X absorbs radiation with a photon energy than the ionizat...
Text Solution
|
- When an atom X absorbs radiation with a photon energy than the ionizat...
Text Solution
|
- When an atom X absorbs radiation with a photon energy than the ionizat...
Text Solution
|
- 16 eV ऊर्जा का फोटॉन हाइड्रोजन परमाणु को मूल-ऊर्जा स्तर में आयनित करता...
Text Solution
|
- हाइड्रोजन परमाणु का आयनन-विभव 13.6 वोल्ट है। निम्नतम ऊर्जा-स्तर में यह...
Text Solution
|