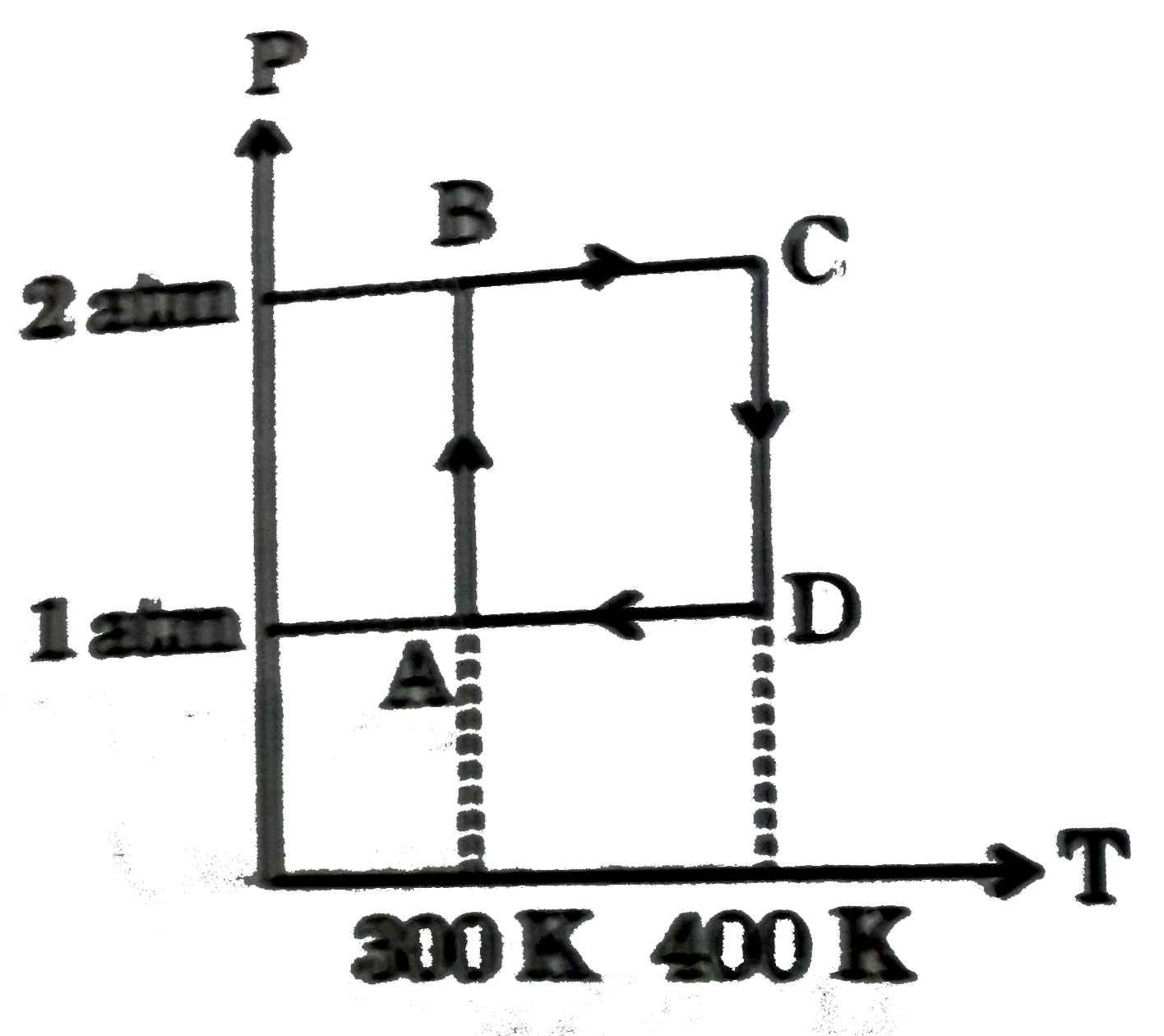
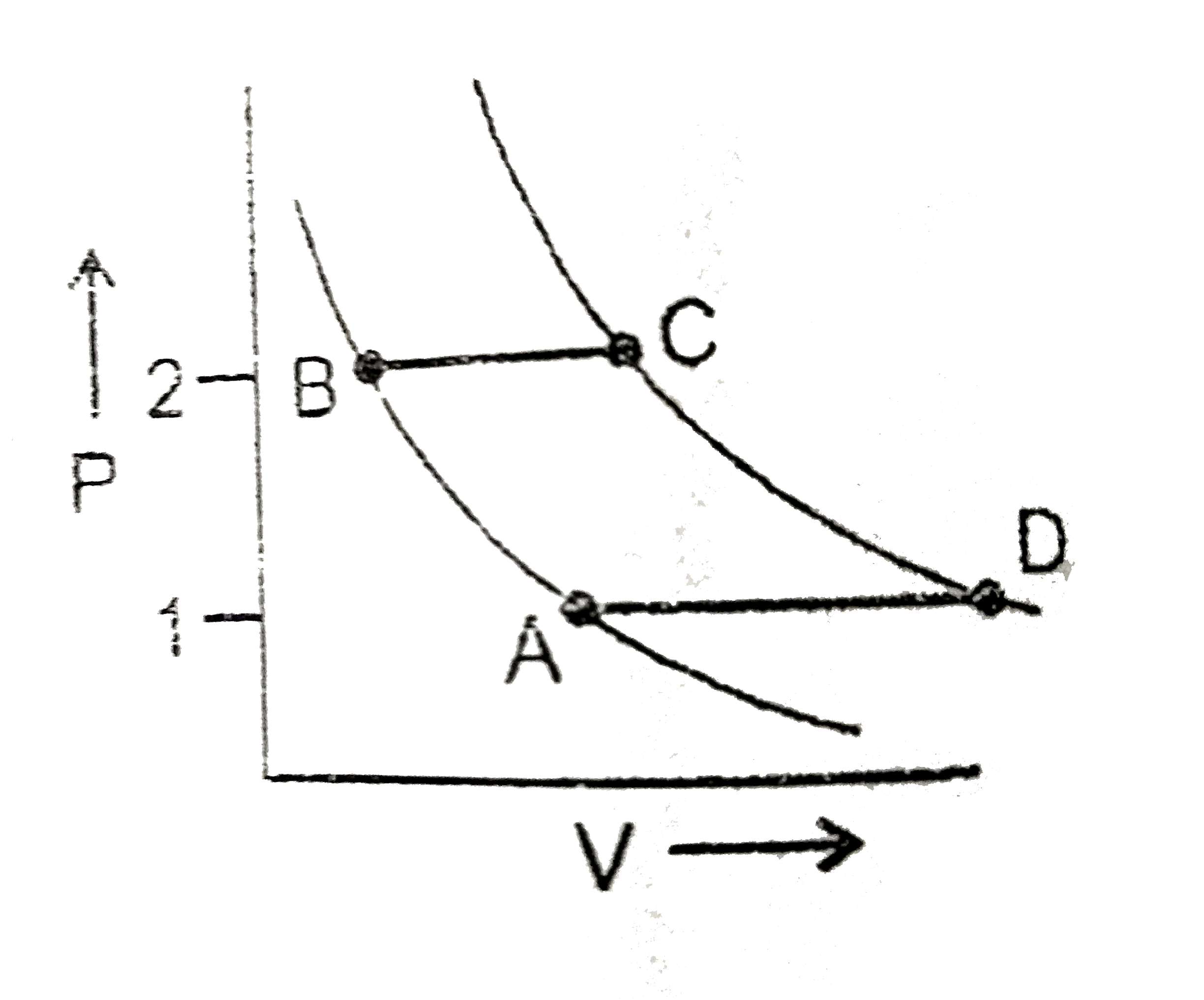
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise-2 II: Single and double value integer type|16 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise 2- III : One or more than one options correct type|14 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise -1 Part -II Only option correct type|92 VideosTEST SERIES
RESONANCE ENGLISH|Exercise CHEMISTRY|50 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-THERMODYNAMICS-Exercise -2 Part-I: Only one option correct type
- The plots between P and V which represent isochoric and isobaric proce...
Text Solution
|
- Consider the cyclic process R rarr S rarr R as shown in the fig. You a...
Text Solution
|
- In the cyclic process shown on V-P diagram, the magnitude of the work ...
Text Solution
|
- w for the following process ABCD on a monoatomic gas are:
Text Solution
|
- A given mass of a gas expands from the state A to the state B by three...
Text Solution
|
- Two moles of Helium gas undergo a reversible cyclic process as shown i...
Text Solution
|
- 50 L of a certain liquid is confined in a piston system at the externa...
Text Solution
|
- For an isobaric process , the ratio of Delta Q (amount of heat supplie...
Text Solution
|
- Ice-water mass ratio is maintntained as 1:1 in a given system conta...
Text Solution
|
- Two mole of an ideal gas is heated at constant pressure of one atmosp...
Text Solution
|
- The increase in internal energy of 1 kg of water at 100^(@) C when it ...
Text Solution
|
- Consider a classroom that is roughly 5mxx10mxx3m. Initially T=27^(@)C ...
Text Solution
|
- A heat engine carries one mole of an ideal monoatomic gas around the c...
Text Solution
|
- Which one of the following equations does not correctly respresents th...
Text Solution
|
- One mole of an ideal gas (C(v,m)=(5)/(2)R) at 300 K and 5 atm is expan...
Text Solution
|
- There are two statement of same gas initially under similar initial st...
Text Solution
|
- There are two statement of same gas initially under similar initial st...
Text Solution
|
- The magnitude of enthalpy changes for irreversible adiabatic expansion...
Text Solution
|
- A new flourocarbon of molar mass 102 g mol^(-1) was placed in an elect...
Text Solution
|
- A certain gas in expanded from (1L, 10 atm) to (4L, 5 atm) against a c...
Text Solution
|