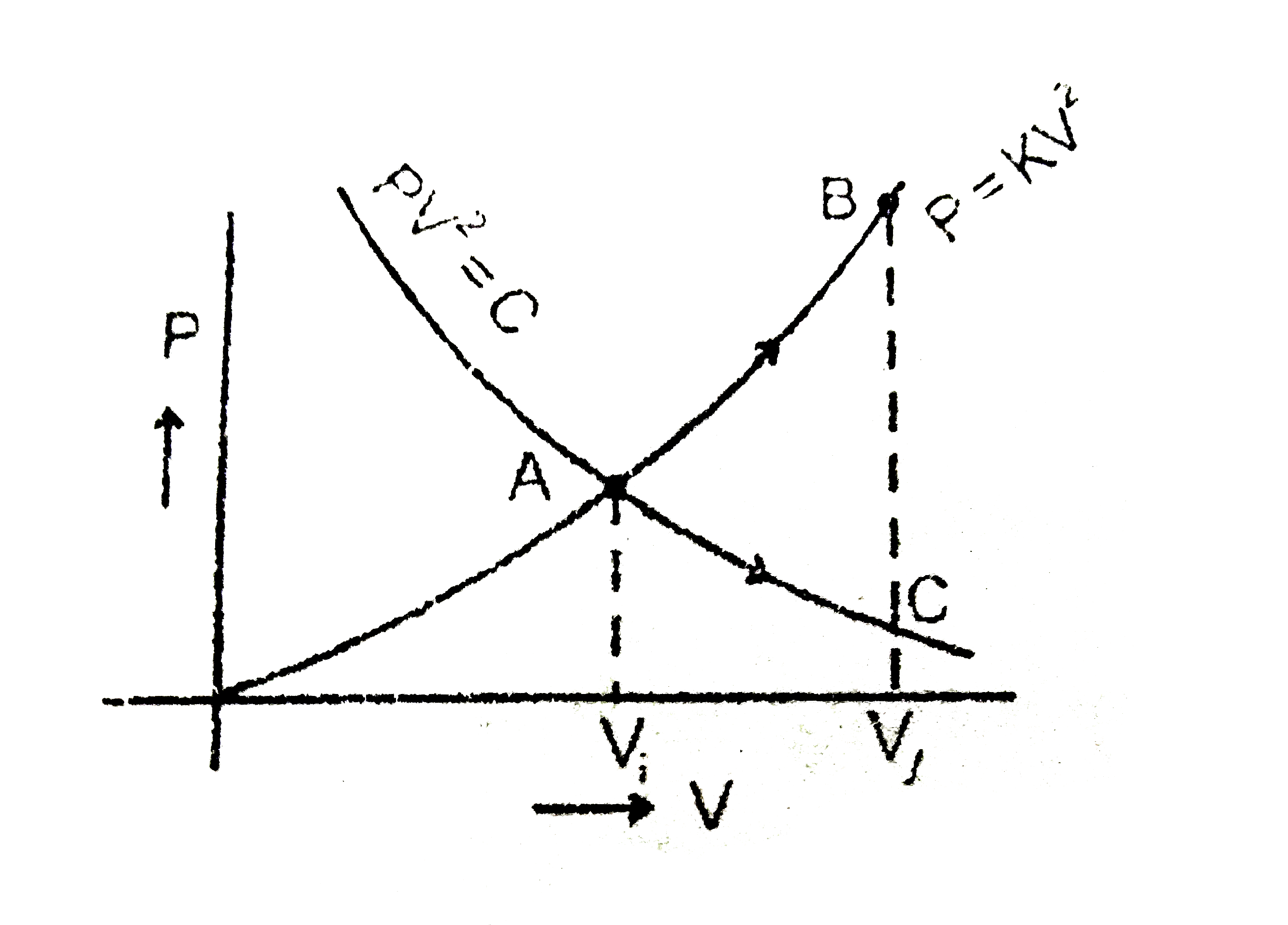A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise-2 part -IV : Comprehension|11 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise-3 Part-1: JEE (ADVANCED)|11 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise-2 II: Single and double value integer type|16 VideosTEST SERIES
RESONANCE ENGLISH|Exercise CHEMISTRY|50 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-THERMODYNAMICS-Exercise 2- III : One or more than one options correct type
- Which of the following properties of system are intensive ?
Text Solution
|
- Which of the following are incorrect ?
Text Solution
|
- Choose the correct statement:
Text Solution
|
- In an isothermal expansion of a gaseous sample the correct relation is...
Text Solution
|
- During the isothermal of an ideal gas :
Text Solution
|
- P-V plot for two gases (assuming ideal) during adiabatic processes are...
Text Solution
|
- When some potential difference V is applied across a resistance R then...
Text Solution
|
- 0.5 mole each of two ideal gasesA (C(v,m)=(5)/(2)R) and B (C(v,m)=3R) ...
Text Solution
|
- 1 mol of NH(3) gas at 27^(@)C is expanded under adiabatic condition to...
Text Solution
|
- 5 moles of a liqiud L are converted into its vapour at its boiling poi...
Text Solution
|
- An ideal gas undergoes adiabatic expansion against constant external p...
Text Solution
|
- Three moles of an ideal diatomic gas undergoes a change in state from ...
Text Solution
|
- Following graph is constructed for the fixed amount of the gas
Text Solution
|
- An ideal gas can be expended from an initial state to a certain volume...
Text Solution
|