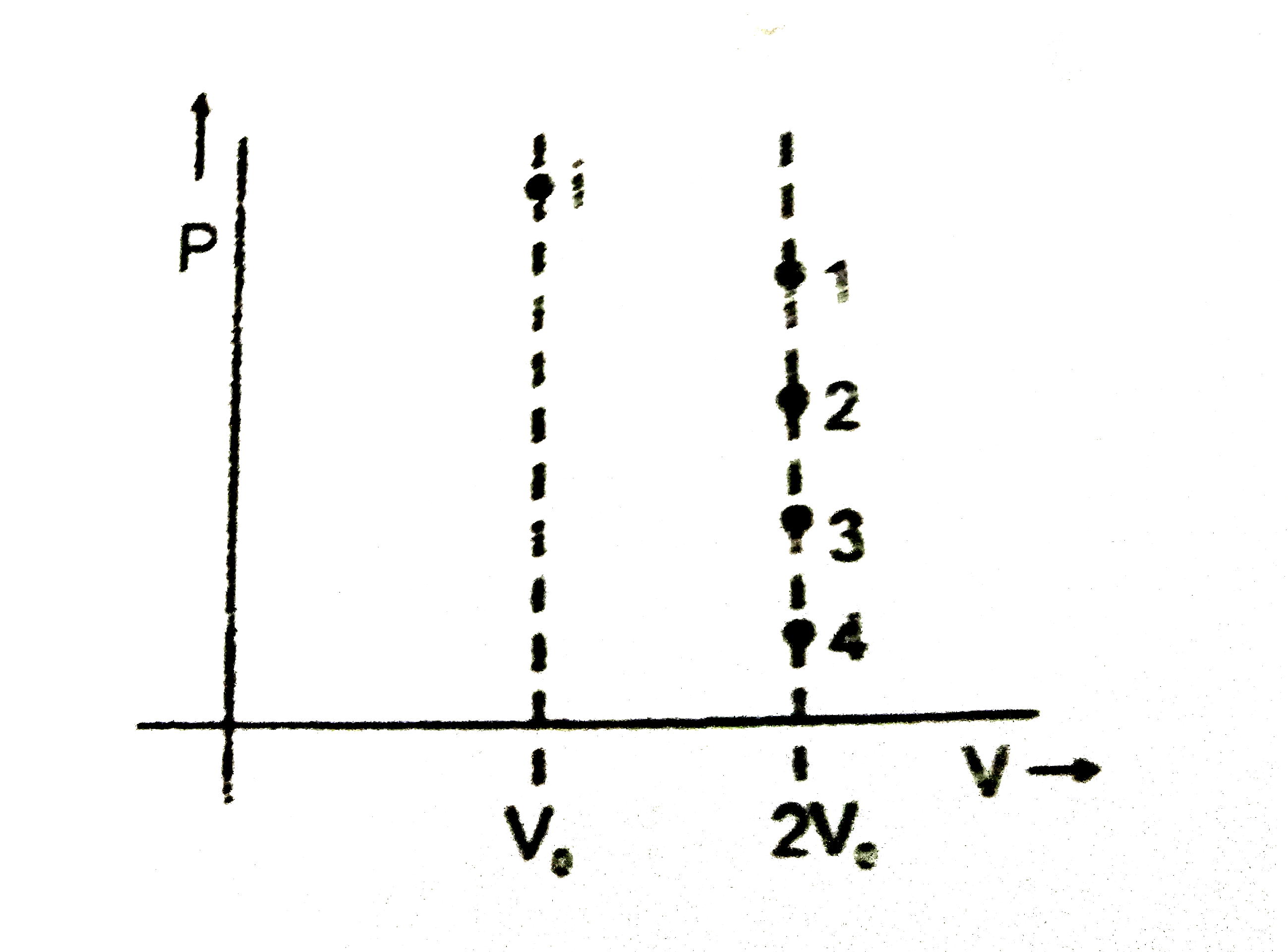A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise-3 Part-1: JEE (ADVANCED)|11 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise Main|5 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise Exercise 2- III : One or more than one options correct type|14 VideosTEST SERIES
RESONANCE ENGLISH|Exercise CHEMISTRY|50 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-THERMODYNAMICS-Exercise-2 part -IV : Comprehension
- When a system is taken from state A to state B along path ACB as shown...
Text Solution
|
- When a system is taken from state A to state B along path ACB as shown...
Text Solution
|
- When a system is taken from state A to state B along path ACB as shown...
Text Solution
|
- A gaseous sample is generally allowed to do only expansion//compressio...
Text Solution
|
- A gaseous sample is generally allowed to do only expansion//compressio...
Text Solution
|
- A gaseous sample is generally allowed to do only expansion//compressio...
Text Solution
|
- A gaseous sample is generally allowed to do only expansion//compressio...
Text Solution
|
- Explain the following in terms of gain of oxygen with two examples for...
Text Solution
|
- Phase transitions are ubiquitous in nature. We are all familiar with t...
Text Solution
|
- Phase transitions are ubiquitous in nature. We are all familiar with t...
Text Solution
|
- Phase transitions are ubiquitous in nature. We are all familiar with t...
Text Solution
|