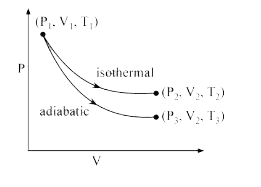
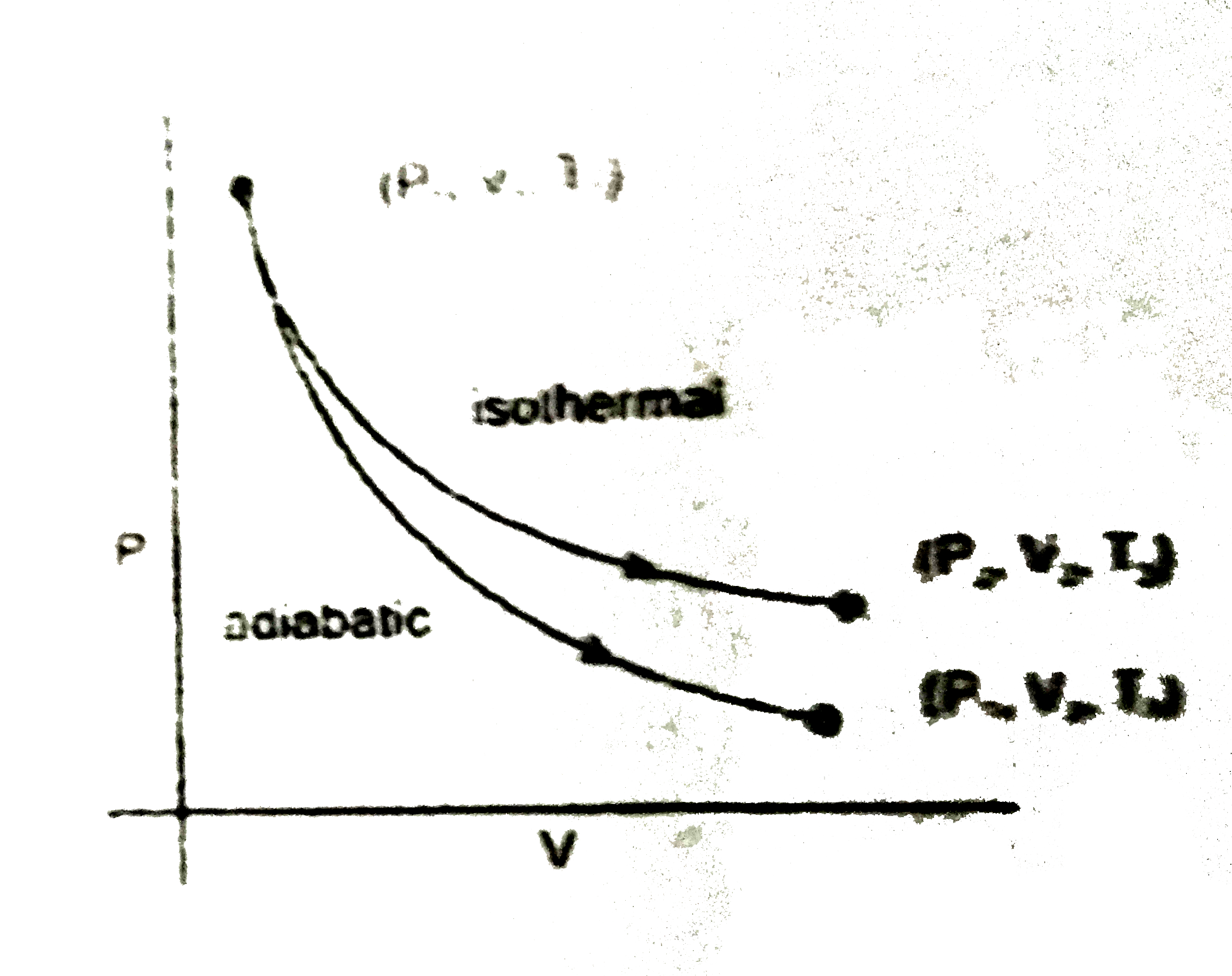A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-THERMODYNAMICS-Exercise-3 Part-1: JEE (ADVANCED)
- C(v) value of He is always 3R//2 but C(v) value of H(2) is 3R//2 at lo...
Text Solution
|
- 2 moles of ideal gas is expanded isothermally & reversibly from 1 litr...
Text Solution
|
- There is 1 mol liquid (molar volume 100 ml) in an adiabatic container ...
Text Solution
|
- One mole of an ideal monoatomic gas at temperature T and volume 1L exp...
Text Solution
|
- The ratio of P to V at any instant is constant and is equal to 1, for ...
Text Solution
|
- For the reaction 2CO +O(2) rarr 2CO(2), DeltaH =- 560 kJ, 2mol of ...
Text Solution
|
- Among the following the intensive properly is (properties are):
Text Solution
|
- Among the following the intensive properly is (properties are):
Text Solution
|
- One mole of an ideal gas is taken from a to b along two paths denoted ...
Text Solution
|
- The reversible expansion of an ideal gas under adiabatic and isotherma...
Text Solution
|
- An ideal gas in a thermally insulated vessel at internal pressure =P(1...
Text Solution
|