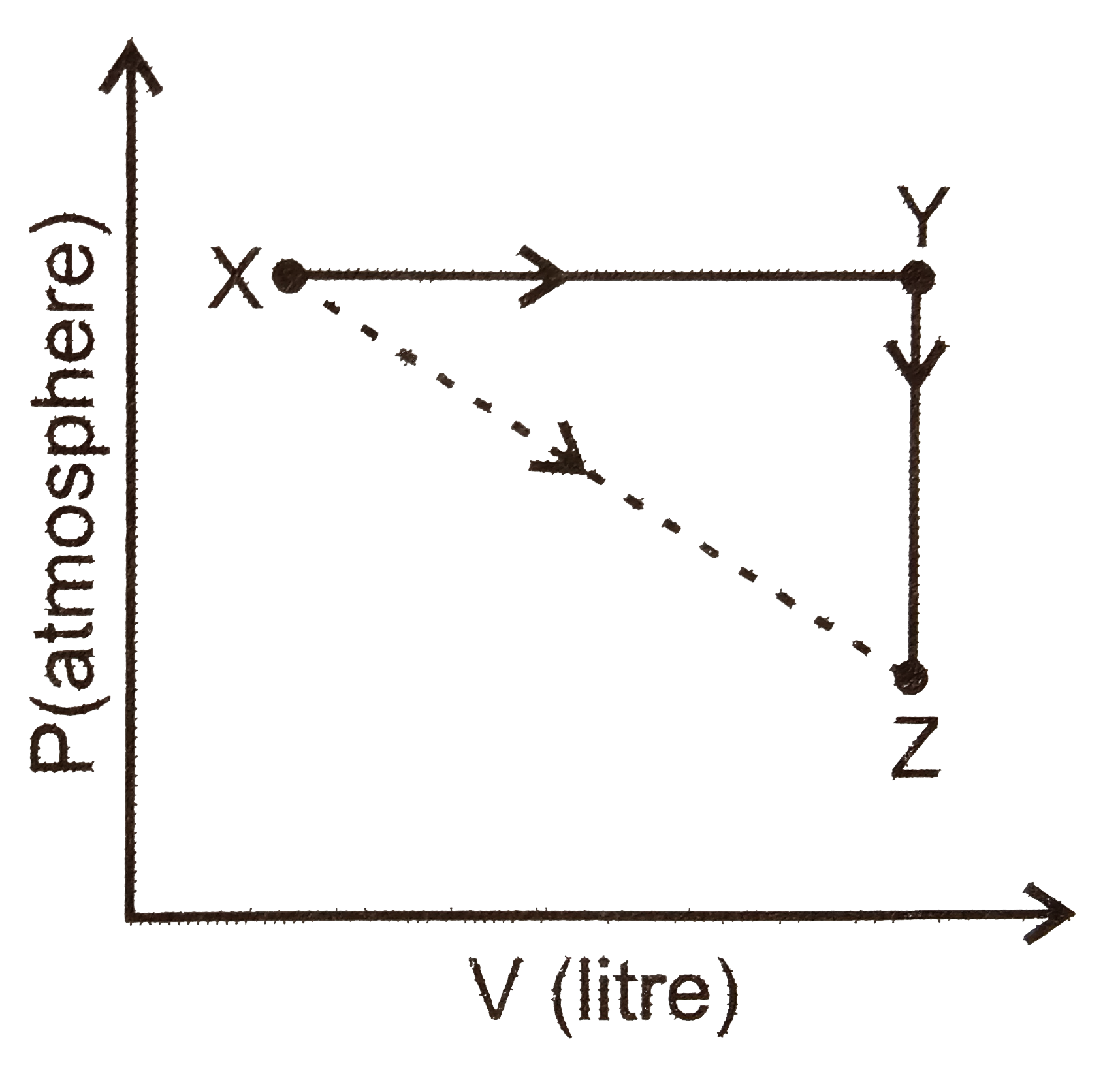
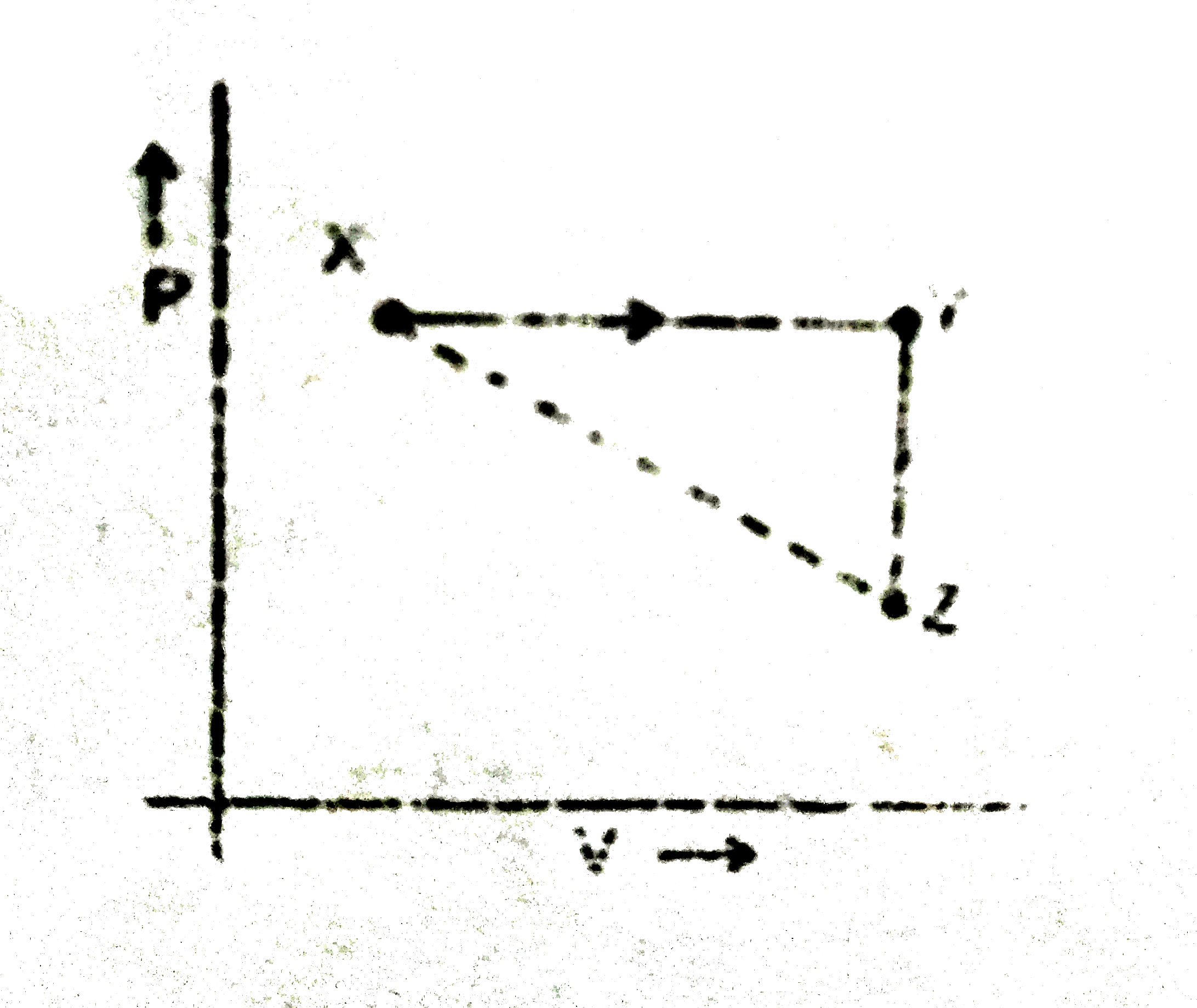
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
RESONANCE ENGLISH|Exercise exercise-3 part-1 Advanced level Solutions|30 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise exercise-3 part-2 Advanced level Solutions|27 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise exercise-2 Part:(IV): comprehension|8 VideosTEST SERIES
RESONANCE ENGLISH|Exercise CHEMISTRY|50 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-THERMODYNAMICS-exercise-3 Part:(I)
- The thermodynamic property that measures the extent of molecular disor...
Text Solution
|
- N(2) + 3H(2) hArr 2NH(3)" "K=4xx 10^(6)"at"298 " ...
Text Solution
|
- The value of log(10)K for a reaction A hArr B is (Given: Delta(f)H(298...
Text Solution
|
- For the process H(2)O(l) (1 "bar", 373 K) rarr H(2)O(g) (1"bar", 373 K...
Text Solution
|
- Statement -1: For every chmical reaction at equilibrium , standard Gid...
Text Solution
|
- Assertion (A) : There is a natural asymmetry between converting work t...
Text Solution
|
- Match the transformation in colums I with appropriate options in colum...
Text Solution
|
- For an ideal gas, consider only P-V work in going from an initial stat...
Text Solution
|
- For the process H(2)O(l)toH(2)O(g) at t=100^(@)C and 1 atmosphere pres...
Text Solution
|
- Match the thermodynamic processes given under column I with the expres...
Text Solution
|
- For a spontaneous reaction, DeltaG, equilibrium constant K and E("cell...
Text Solution
|
- Identify the correct statement regarding a spontaneous process:
Text Solution
|
- In conversion of limestone to lime , CaCO3(s) rarrCaO(s)+CO2(g) the va...
Text Solution
|
- Standard entropies of X(2), Y(2) and XY(3) are 60, 40 and 50 JK^(–1) m...
Text Solution
|
- In a fuel cell methanol is used as fuel and oxygen gas is used as an o...
Text Solution
|
- For a particular reversible reaction at temperature T, DeltaH and Delt...
Text Solution
|
- The entropy change involved in the isothermal reversible expansion of ...
Text Solution
|
- In view of the signs of DeltarG^@ for the following reactions PbO2 +...
Text Solution
|
- The incorrect expression among the following is
Text Solution
|
- The standard free energy of formation of NO(g) is 86.6 kJ/ mol at 29...
Text Solution
|