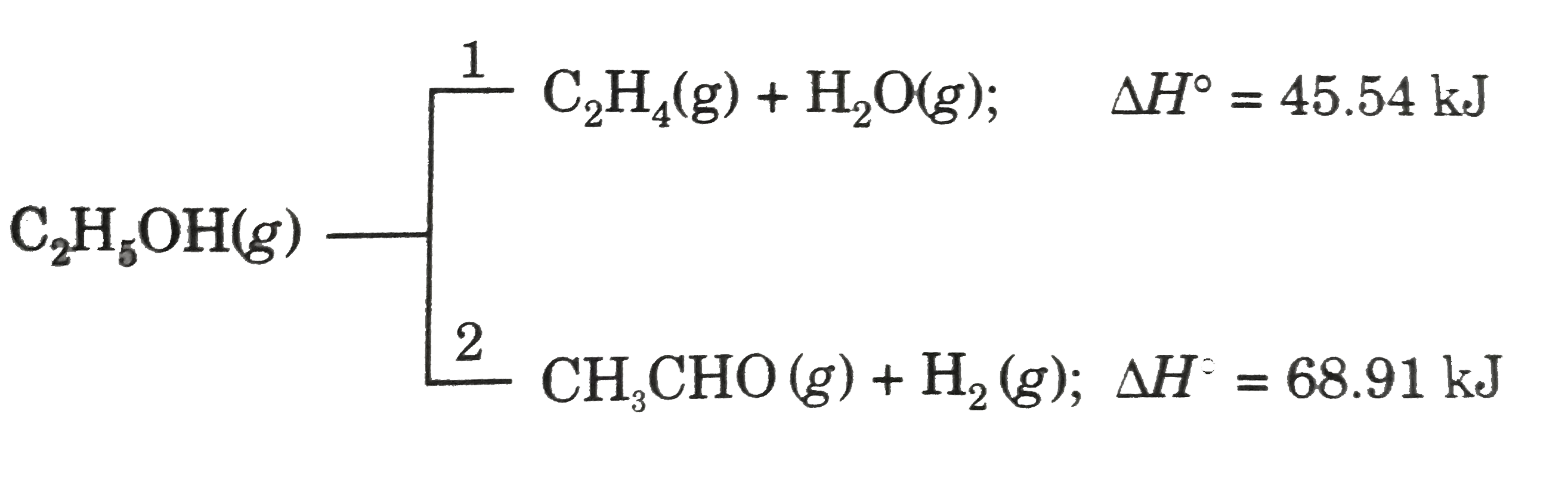A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
RESONANCE ENGLISH|Exercise exercise-3 part-2 Advanced level Solutions|27 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise exercise-3 part-III Advanced level Solutions (STAGE-I)|6 VideosTHERMODYNAMICS
RESONANCE ENGLISH|Exercise exercise-3 Part:(I)|23 VideosTEST SERIES
RESONANCE ENGLISH|Exercise CHEMISTRY|50 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-THERMODYNAMICS-exercise-3 part-1 Advanced level Solutions
- Ethyl chloride (C(2)H(5)Cl) , is prepared by reaction of ethylene with...
Text Solution
|
- Which statement regarding entropy is correct?
Text Solution
|
- One mole of solid Zn is placed in excess of dilute H(2)SO(4) at 27^(@)...
Text Solution
|
- The enhtaply change for the reaction of 50ml of ethylene with 50.0 mL ...
Text Solution
|
- When 1 mol of ice melts at 0^(@)C and a constant pressure of 1 atm, 14...
Text Solution
|
- One mole of ideal monoatmic gas is carried throught the reversible cyc...
Text Solution
|
- 130 g of Zn is dissolved in dilute sulphuric acid in an open beaker . ...
Text Solution
|
- The enthalpy of combustion of propane (C(3)H(8)) gas in terms of given...
Text Solution
|
- If x1, x2 and x3 are enthalpies of H - H, O = O, O - H bonds respect...
Text Solution
|
- NH(3)(g) + 3Cl(2)(g) rarr NCl(3)(g) + 3HCl(g), " "DeltaH(1) N(2...
Text Solution
|
- The enthalpy of formation of H(2)O(l) is -285 KJ mol^(-1) and enthalpy...
Text Solution
|
- Ethanol can undergo decompostion to form two sets of products. If th...
Text Solution
|
- Find bond enthalpy of S-S bond from the following data: C(2)H(5)-S-C...
Text Solution
|
- From given following equations and DeltaH^(@) values, determine the en...
Text Solution
|
- Animals operate under conditions of constant pressure and most of the ...
Text Solution
|
- From the given table answer the following question: {:(,CO(g),CO(2)(...
Text Solution
|
- Calculate the free energy charge at 298 K for reaction Br(2)(l) + Cl(2...
Text Solution
|
- One gram sample of oxygen undergoes free expansion from 0.75L to 3.0 L...
Text Solution
|
- Given that: DeltaG(F)^(@)(CuO) =-30.4 "Kcal"//"mole" DeltaG(f)^(@)...
Text Solution
|
- Calculate Delta(f)G^(@) " for "(NH(4)Cl,s) at 310 K. Given : Delta(f...
Text Solution
|