Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-THERMODYNAMICS-exercise-3 part-III Advanced level Solutions (STAGE-II)
- The rapid depletion of fossil fuels has inspired extensive research ...
Text Solution
|
- One method to produce hydrogen on an industrial on an industrial scale...
Text Solution
|
- One method to produce hydrogen on an industrial on an industrial scale...
Text Solution
|
- A heat engine is a system that converts heat into mechanical work. A h...
Text Solution
|
- A heat engine is a system that converts heat into mechanical work. A h...
Text Solution
|
- A heat engine is a system that converts heat into mechanical work. A h...
Text Solution
|
- Oxygen is of vital importance for all of us . Oxygen enters the body v...
Text Solution
|
- Since 1891 lighting lamps have been manufactured in the Netherlands. T...
Text Solution
|
- Since 1891 lighting lamps have been manufactured in the Netherlands. T...
Text Solution
|
- Since 1891 lighting lamps have been manufactured in the Netherlands. T...
Text Solution
|
- A very large swimming pool filled with water of temperture equal to 20...
Text Solution
|
- A very large swimming pool filled with water of temperture equal to 20...
Text Solution
|
- A very large swimming pool filled with water of temperture equal to 20...
Text Solution
|
- A very large swimming pool filled with water of temperture equal to 20...
Text Solution
|
- A very large swimming pool filled with water of temperture equal to 20...
Text Solution
|
- For his 18th birthday in February Peter plants to turn a hut in the ga...
Text Solution
|
- For his 18th birthday in February Peter plants to turn a hut in the ga...
Text Solution
|
- For his 18th birthday in February Peter plants to turn a hut in the ga...
Text Solution
|
- For his 18th birthday in February Peter plants to turn a hut in the ga...
Text Solution
|
- For his 18th birthday in February Peter plants to turn a hut in the ga...
Text Solution
|
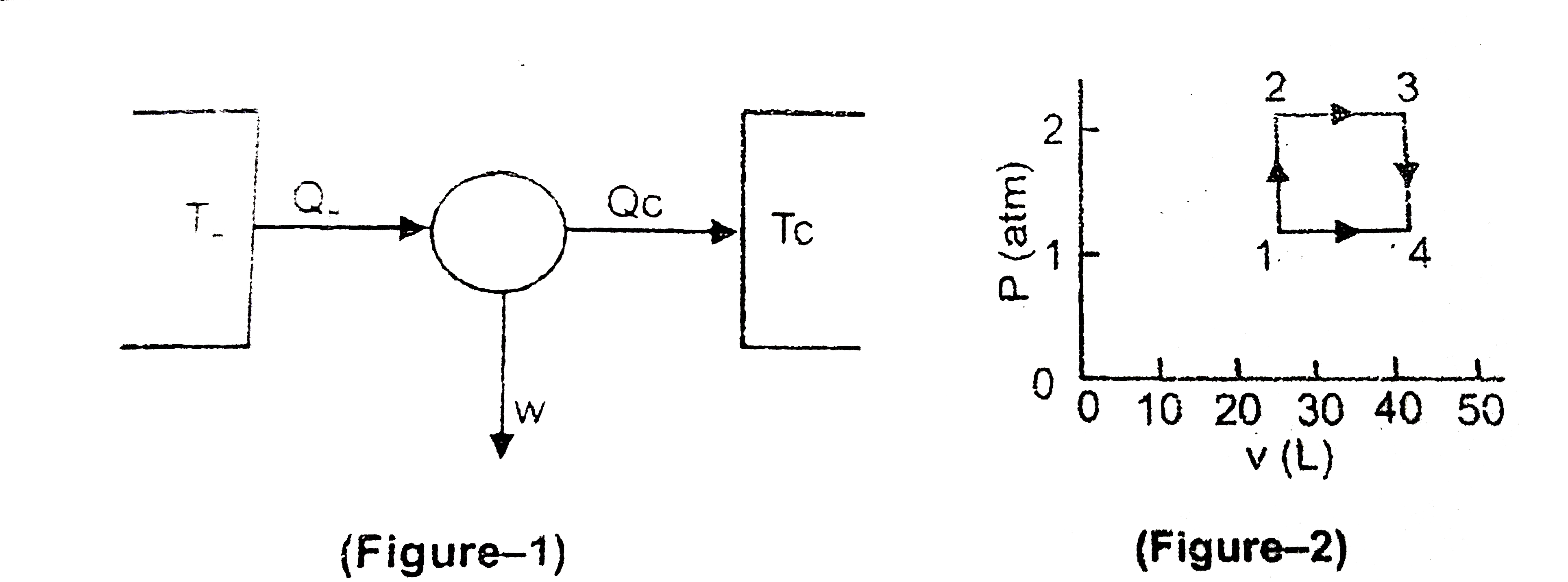 Heat engines can be modelled using themodynamic cycles. The heat engine given in Figure -2 is of a working substance which is 1.00 mol of a monoatomic ideal gas . The thermodynamic cycle begins at the point designated as '1' and goes clockwise and the values of P and l or V at each point is as given below Fig-2 `(P_(1)=1.00 atm " and " V_(1) = 24.6 L , P_(2)=2.00 atm , V_(3) =49.2L, P_(4)=1.00 atm)`
Heat engines can be modelled using themodynamic cycles. The heat engine given in Figure -2 is of a working substance which is 1.00 mol of a monoatomic ideal gas . The thermodynamic cycle begins at the point designated as '1' and goes clockwise and the values of P and l or V at each point is as given below Fig-2 `(P_(1)=1.00 atm " and " V_(1) = 24.6 L , P_(2)=2.00 atm , V_(3) =49.2L, P_(4)=1.00 atm)`