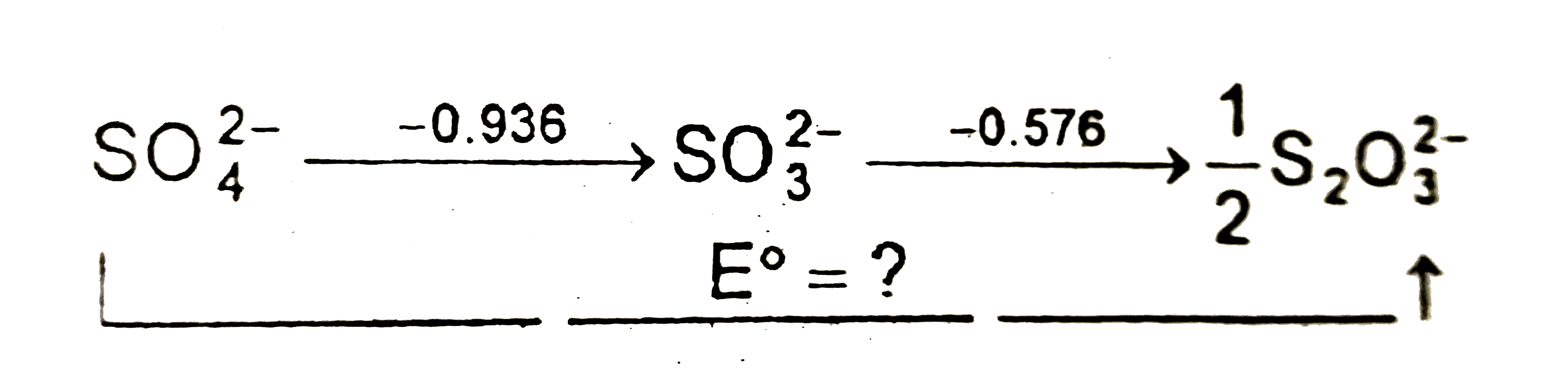Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Assertion Reasoning|11 VideosELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Subjective Questions|14 VideosELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Board Level Exercise|20 VideosELECTRO CHEMISTRY
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (ELECTROCHEMISTRY)|53 VideosEQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise Part -IV|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ELECTROCHEMISRY-Exercise
- If E^(o)""(Fe^(+2)//Fe) is x(1),E^(o)""(Fe ^(+3)//Fe)is x(2), then E^(...
Text Solution
|
- The standard reduction potential of TiO^(2+) and Ti^(3+) are given by ...
Text Solution
|
- consider the standard reduction potentials (in volts) as shown in figu...
Text Solution
|
- The standard oxidation potentials for Mn^(3+) ion acid solution are Mn...
Text Solution
|
- Using the DeltaG^(@) for the reactions {:(C+O(2)rarrCO(2),DeltaG^(@...
Text Solution
|
- Make complete cell diagrams of the following cell reactions (a). Cd^...
Text Solution
|
- Write cell reaction of the following cells: (a). Pt|Fe^(2+),Fe^(3+)|...
Text Solution
|
- The oxidation potential of a hydrogen electrode at pH = 1 is (T = 298 ...
Text Solution
|
- The standard oxidation potential for the half-cell NO(2)^(-)(g)+H(2)...
Text Solution
|
- In a reaction Cr(2)O(7)^(2-) is reduced to Cr^(3+). What will be the c...
Text Solution
|
- The standard reduction potential for Cu^(2+)|Cu is +0.34V. Calculate t...
Text Solution
|
- The EMF of the cell M|M^(n+)(0.02M) ||H^(+)(1M)|H(2)(g) (1atm)Pt at 25...
Text Solution
|
- Consider the following electrochemical cell. (a). Write a balanced n...
Text Solution
|
- An electrochemical cell is constructed with an open switch as shown be...
Text Solution
|
- Equinormal solution of two weak acids, HA(pK(a) =3) and HB(pK(a) =5) a...
Text Solution
|
- In two vessels each containing 500mL water, 0.05m mol of aniline (K(b)...
Text Solution
|
- Given : NO(3)^(-) rarrNO(2)( acidic medium ), " "E^(-)=0.8V N...
Text Solution
|
- The standard oxidation potential of Zn referred to SHE is 0.76V and th...
Text Solution
|
- The e.m.f of cell Ag|AgI((s)),0.05M KI|| 0.05 M AgNO(3)|Ag is 0.788 V....
Text Solution
|
- The cell Pt, H(2) (1atm) H^(+) (pH =x)| Normal calomel Electrode has a...
Text Solution
|