Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Assertion Reasoning|11 VideosELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Subjective Questions|14 VideosELECTROCHEMISRY
RESONANCE ENGLISH|Exercise Board Level Exercise|20 VideosELECTRO CHEMISTRY
RESONANCE ENGLISH|Exercise PHYSICAL CHEMITRY (ELECTROCHEMISTRY)|53 VideosEQUIVALENT CONCEPT & TITRATIONS
RESONANCE ENGLISH|Exercise Part -IV|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ELECTROCHEMISRY-Exercise
- The standard reduction potential for Cu^(2+)|Cu is +0.34V. Calculate t...
Text Solution
|
- The EMF of the cell M|M^(n+)(0.02M) ||H^(+)(1M)|H(2)(g) (1atm)Pt at 25...
Text Solution
|
- Consider the following electrochemical cell. (a). Write a balanced n...
Text Solution
|
- An electrochemical cell is constructed with an open switch as shown be...
Text Solution
|
- Equinormal solution of two weak acids, HA(pK(a) =3) and HB(pK(a) =5) a...
Text Solution
|
- In two vessels each containing 500mL water, 0.05m mol of aniline (K(b)...
Text Solution
|
- Given : NO(3)^(-) rarrNO(2)( acidic medium ), " "E^(-)=0.8V N...
Text Solution
|
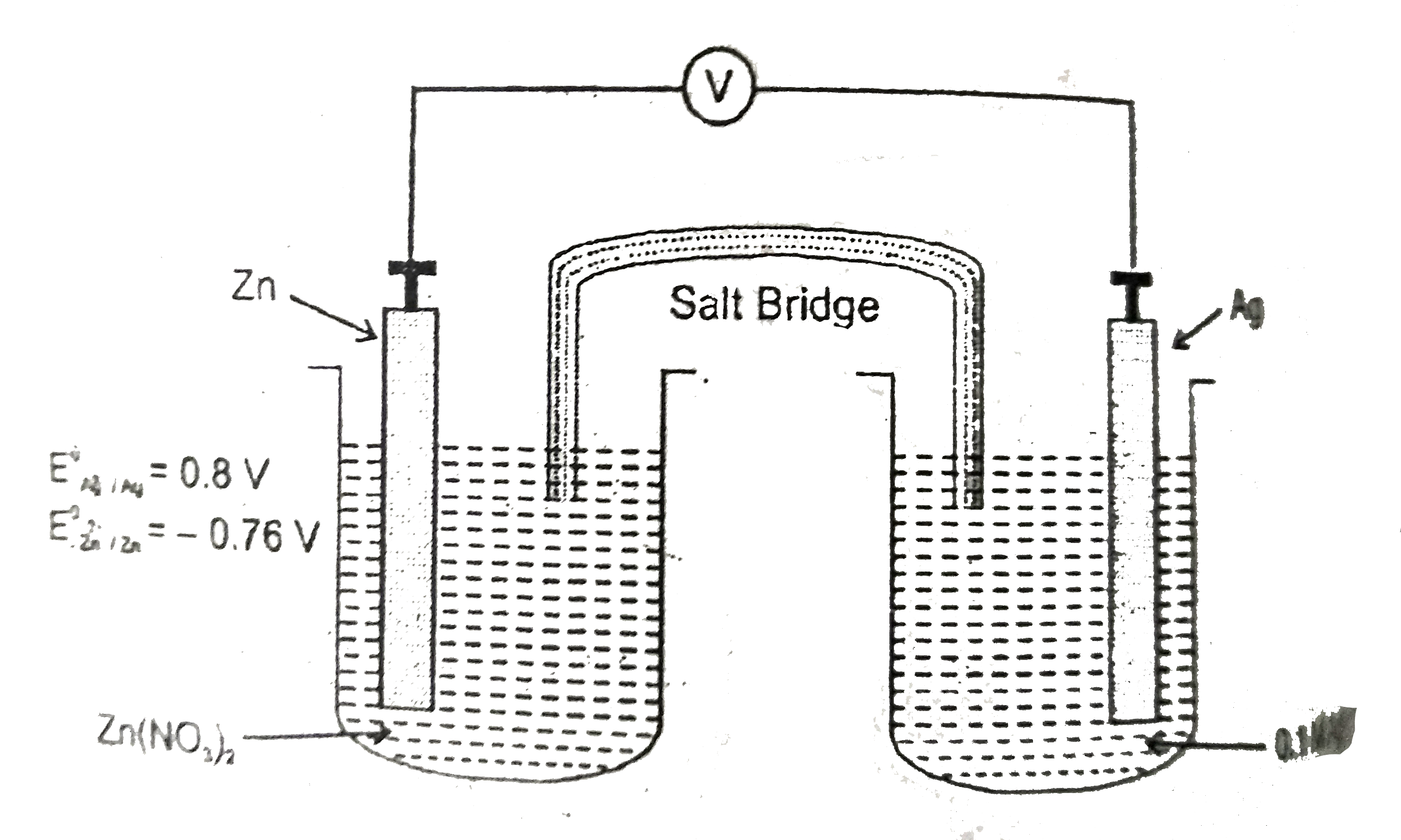
- The standard oxidation potential of Zn referred to SHE is 0.76V and th...
Text Solution
|
- The e.m.f of cell Ag|AgI((s)),0.05M KI|| 0.05 M AgNO(3)|Ag is 0.788 V....
Text Solution
|
- The cell Pt, H(2) (1atm) H^(+) (pH =x)| Normal calomel Electrode has a...
Text Solution
|
- The EMF of the standard weston cadmium cell Cd(12.5%) in Hg|3CdSO(4)...
Text Solution
|
- triangleH for the reactio Ag(s)+(1)/(2)Hg(2)Cl(2)(s)toAgCo(s)+Hg(l) is...
Text Solution
|
- the standart emf of the cel Fe|Fe^(+2)(aq)||Cd^(+2)|Cd is 0.0372 V a...
Text Solution
|
- The voltage of a certain cell has standard potential at 25^(@)C and 20...
Text Solution
|
- Find the number of electrons involved in the electro-deposition of 63....
Text Solution
|
- A current 0.5 ampere when passed through AgNO(3) solution for 193 sec....
Text Solution
|
- A certain metal salt solution is electrolysed in series with a silver ...
Text Solution
|
- 3A current was passed through an aqueous solution of an unknown salt o...
Text Solution
|
- How long a current of 2 A has to be passed through a solution of AgNO(...
Text Solution
|
- A metal is know to form fluoride MF(2). When 10A of electricity is pas...
Text Solution
|