Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ELECTROCHEMISRY-CBSE Problems
- A copper - silver cell is set up . The copper ion concentration in it...
Text Solution
|
- (a) Corrosion is essentially an electrochemical phenomenon. Explain th...
Text Solution
|
- Calculate the cell e.m.f. and DeltaG for the cell reaction at 298K for...
Text Solution
|
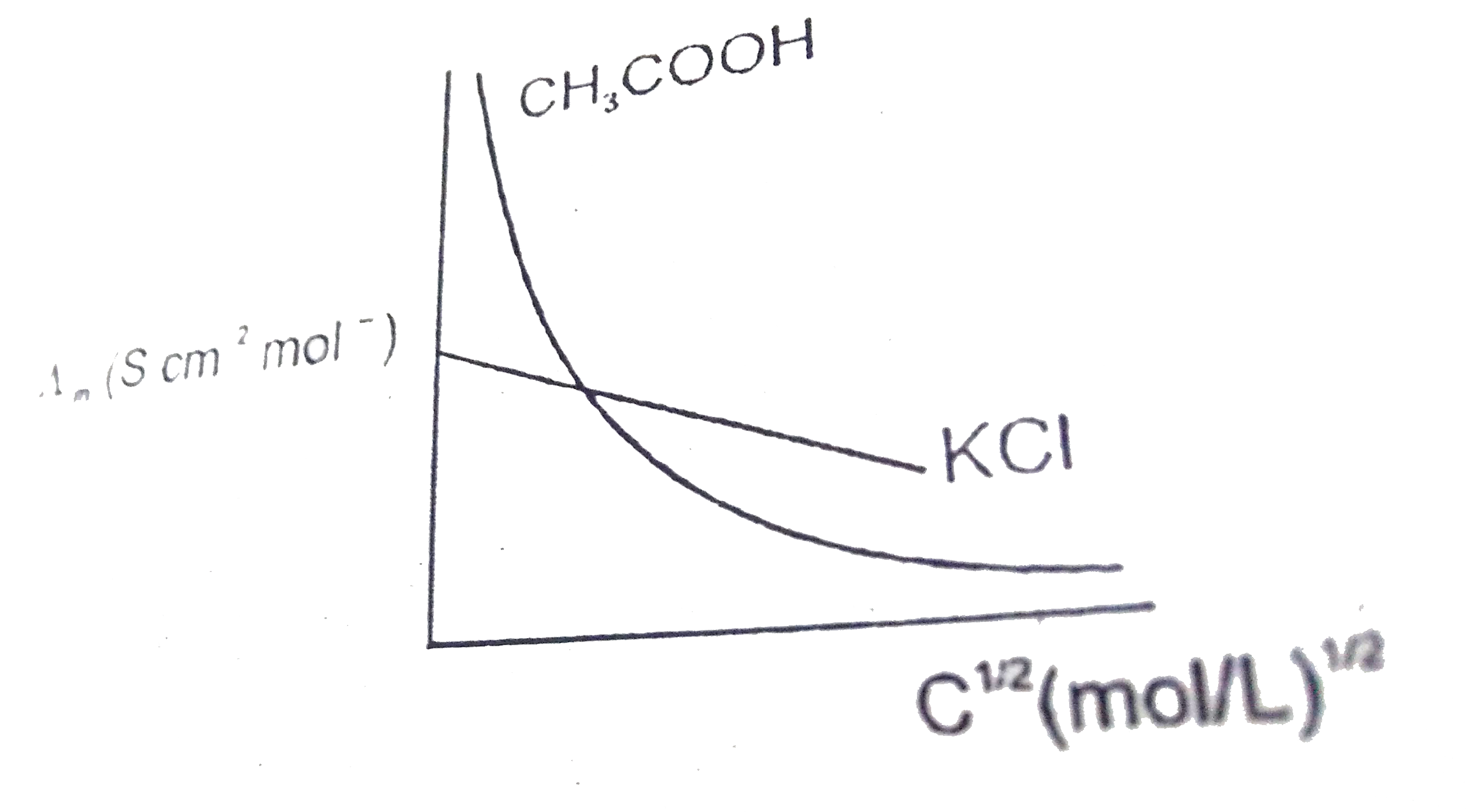
- What is meant by limiting molar conductivity?
Text Solution
|
- Given that the standard electrode (E^(@)) of metals are : K^(+)//K=-...
Text Solution
|
- Express the relation among cell constant , resistance of the solution ...
Text Solution
|
- Two half cell reactions of an electrochemical cell are given below : ...
Text Solution
|
- Definie conductivity and molar conductivity for the solution of an ele...
Text Solution
|
- A copper - silver cell is set up. The copper ion concentrations is 0.1...
Text Solution
|
- Express the relation between.conductivity and molar conductivity of a ...
Text Solution
|
- The chemistry of corrosion of iron is essentially an electrochemical p...
Text Solution
|
- Determine the value of equilibrium constant and for the following ...
Text Solution
|
- What type of a battery is lead storage battery? Write the anode and c...
Text Solution
|
- (a) How many mole of mercury will be produced by electrolysing 1.0 M ...
Text Solution
|
- The molar conductivity of a 1-5 M solution of an electrolyte is found ...
Text Solution
|
- The electrical resistance of a column of 0.05 M NaOH solution of diame...
Text Solution
|
- (a) What type of a battery is the lead storage battery ? Write the ano...
Text Solution
|
- (a) Define molar conductivity of a solution and explain how molar cond...
Text Solution
|
- The conductivity of 0.20 M solution of KCl at 298 k is 0.023 S cm^(-1)...
Text Solution
|
- Calculate the emf of the following cell at 298K: Fe(s) abs(Fe^(2+)(0...
Text Solution
|