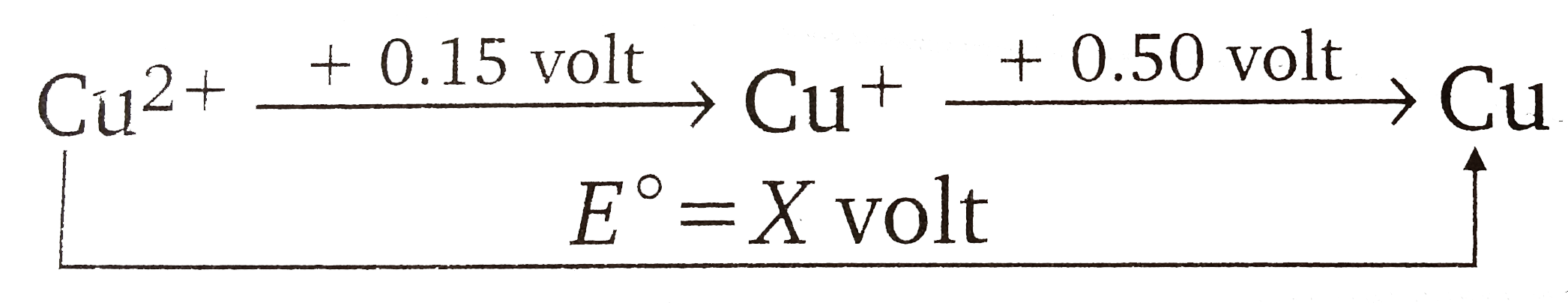Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ELECTROCHEMISRY-Advanced Level Problems
- 10g fairly concentrated solution of CuSO(4) is electrolyzed using 0.01...
Text Solution
|
- An electric current is passed through electrolytic cells in series one...
Text Solution
|
- After electrlysis of NaCI solution with inert electrodes for a certain...
Text Solution
|
- Same quantity of electricity is being used to liberate iodide (at anod...
Text Solution
|
- The resistance of two electrolytes X and Y ere found to be 45 and 100 ...
Text Solution
|
- For 0.0128 N solution fo acetic at 25^(@)C equivalent conductance of t...
Text Solution
|
- Specific conductance of pure water at 25^(@)C is 0.58 xx 10^(-7) mho c...
Text Solution
|
- The reduction potential diagram for Cu in acid solution is : Calc...
Text Solution
|
- For the cellls in opposition, Zn(s)|ZnCl(2)(sol.)|AgCl(s)|Ag|AgCl(s)...
Text Solution
|
- Prove that Moment of couple= Force times couple arm
Text Solution
|
- Why zinc coating protects iron more effectively than tin coating ? ...
Text Solution
|
- For the cell (at 1bar H(2) pressure) Pt|H(2)(g)| HX(m(1)), NaX(m(2)), ...
Text Solution
|
- Delta(f)H^(Theta) per mole of NH(3)(g). NO(g), and H(2)O(l) are -11.04...
Text Solution
|
- In a galvanic cell, the half-cell having more standard potential serve...
Text Solution
|
- At 300 K specific conductivity of ethanol is 4xx10^(-10)mhocm^(-1). Th...
Text Solution
|
- Metallic sodium cannot be prepared from electrolysis of an aqueous sol...
Text Solution
|
- The process of AgCN+KCNtoK[Ag(CN)(2)] involves the oxidation of Ag.
Text Solution
|
- Cations move towards cathode and anions towards anode in both galvanic...
Text Solution
|
- State the following statement is true or false. A positive half-cel...
Text Solution
|
- State True or False Metallic anodes more reactive than platinum tend ...
Text Solution
|