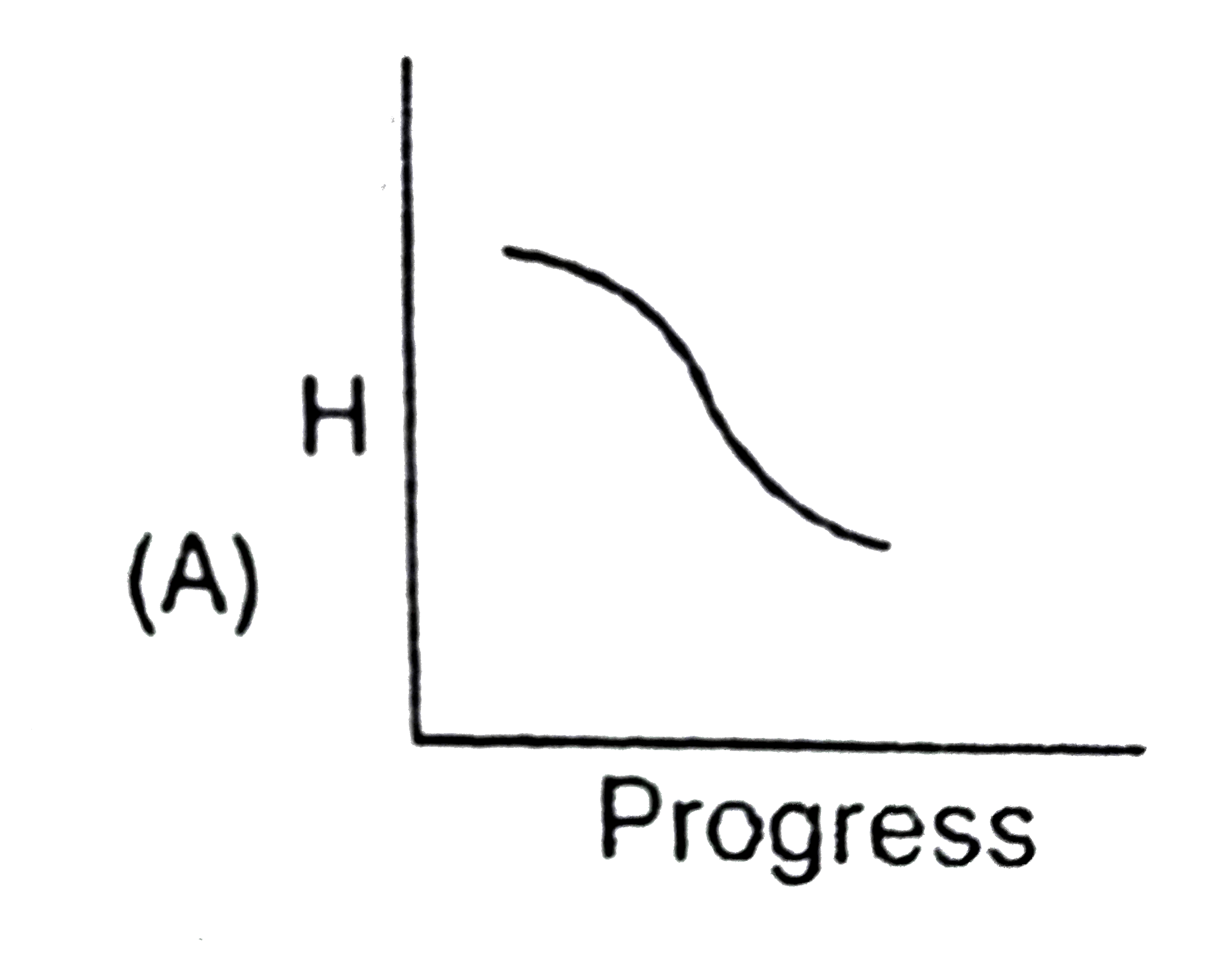
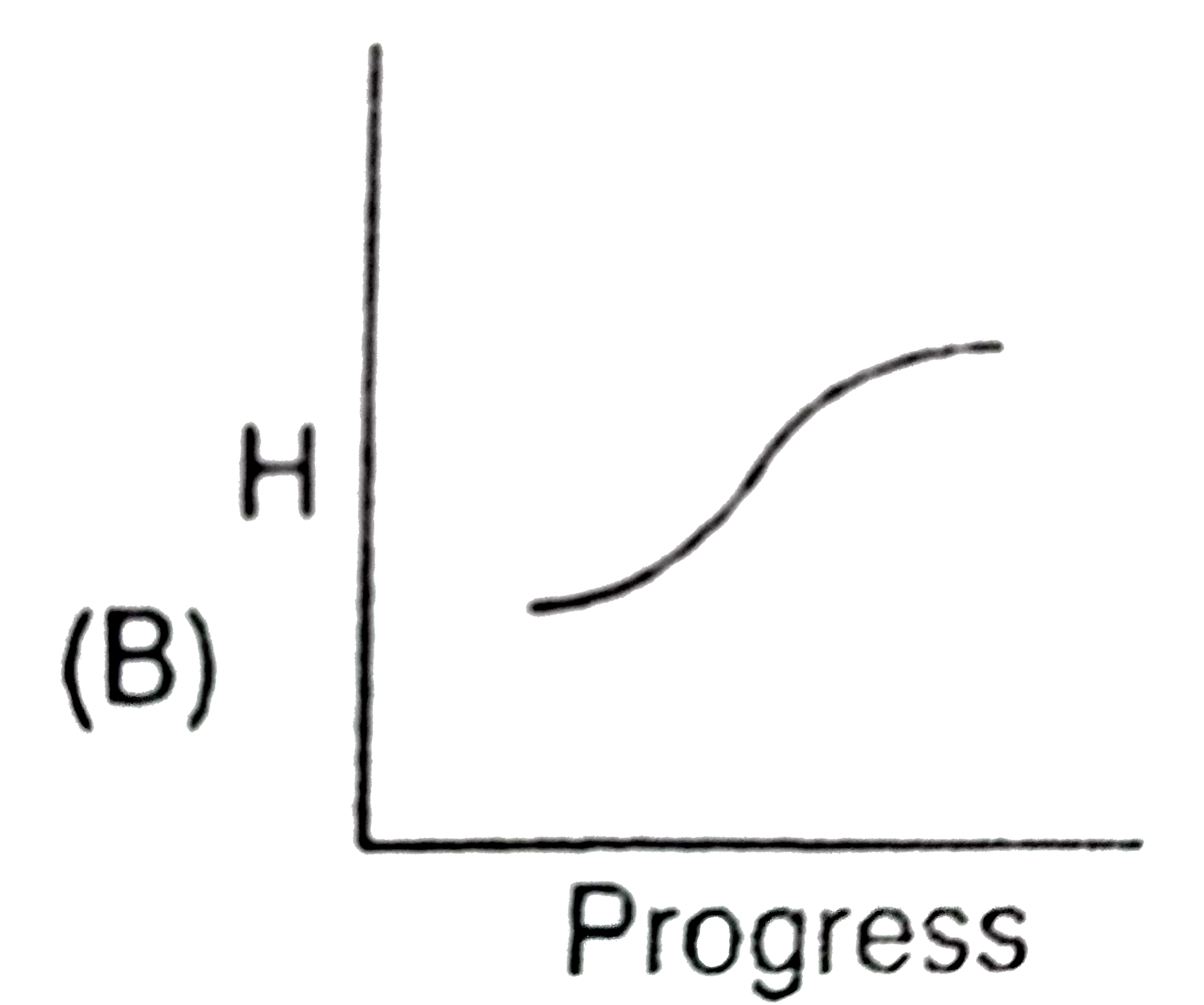
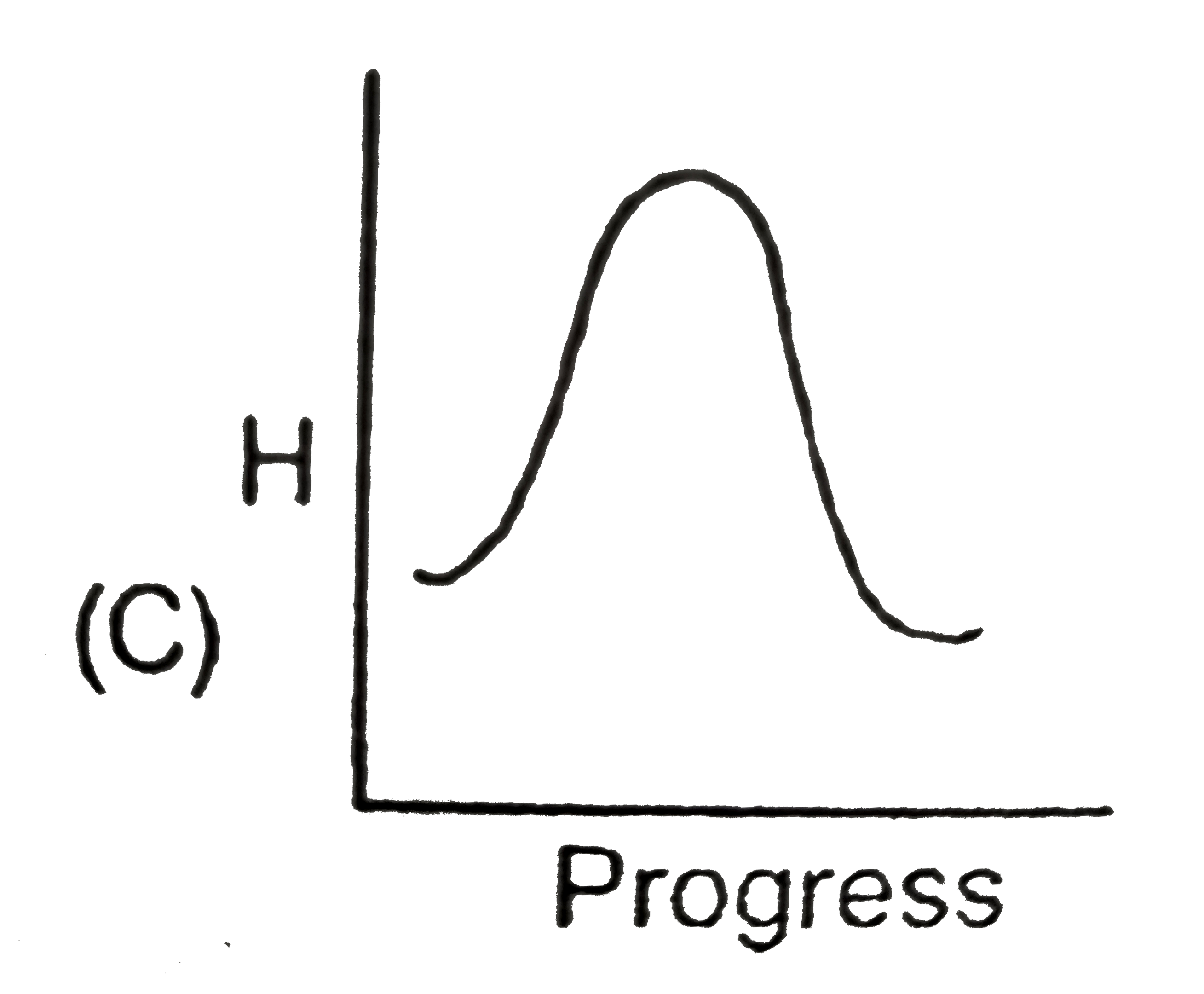
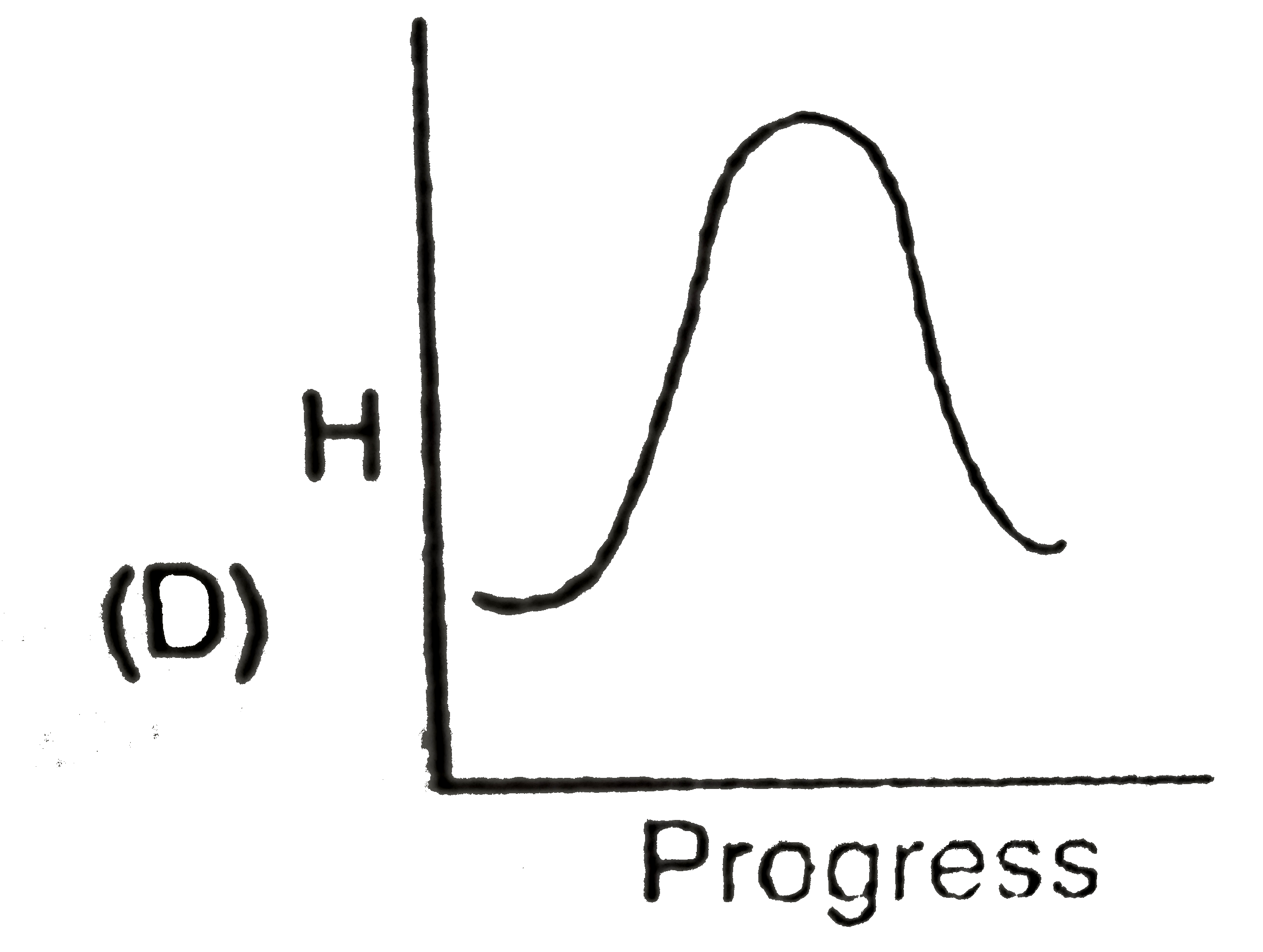
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Identify the appropriate graph between enthalpy and progress of physic...
Text Solution
|
- Identify the appropriate graph between enthalpy and progress of physic...
Text Solution
|
- भौतिक अधिशोषण एवं रासायनिक अधिशोषण में क्या अंतर हैं?
Text Solution
|
- रासायनिक तथा भौतिकी अधिशोषण प्रक्रियाओ में से किसके लिए अधिशोषण ऐन्थेल...
Text Solution
|
- भौतिक अधिशोषण तथा रासायनिक अधिशोषण में क्या अन्तर है ?
Text Solution
|
- Assertion : In physical adsorption , enthalpy of adsorption in very lo...
Text Solution
|
- भौतिक अधिशोषण एवं रसतानिक अधिशोषण में क्या अंतर है?
Text Solution
|
- Out of physisorption and chemisorption which one has lower enthalpy of...
Text Solution
|
- भौतिक अधिशोषण में एन्थैल्पी परिवर्तन की मात्रा रासायनिक अधिशोषण की तुल...
Text Solution
|