A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Advanced Level Problems (Part-3)(Stage-2)|3 VideosCHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Advanced Level Problems (Part-3)(Stage-5)|2 VideosCHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Advanced Level Problems (Part-2)(Section-3)|6 VideosCHEMICAL BONDING
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Fundamental Concept )|6 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE ENGLISH|Exercise Match the column|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-CHEMICAL EQUILIBRIUM-Advanced Level Problems (Part-3)(Stage-1)
- The equilibrium constant (K) for the reaction. A+2BhArr2C+D is:
Text Solution
|
- A solid mixture(5.000 g) consisting of lead nitrate and sodium nitrate...
Text Solution
|
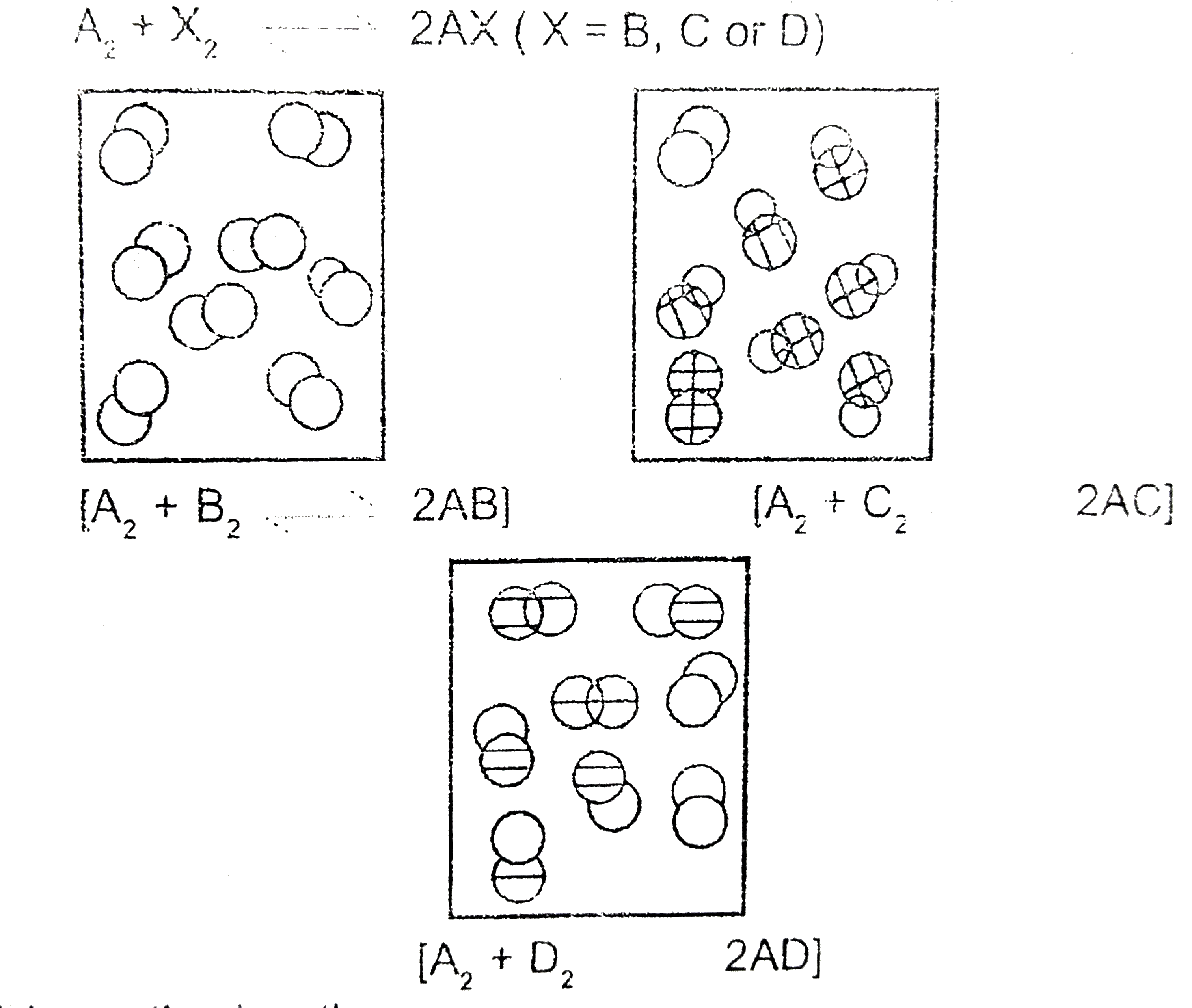
- The following pictures represents the equilibrium state for three diff...
Text Solution
|
- Methanol (CH(3)OH) is manufactured by reaction of carbon monoxide with...
Text Solution
|
- For the reversible reaction,A+BhArrC, the specific reaction rates for ...
Text Solution
|
- The equilibrium constant for the gaseous reaction H(2)+Cl(2)hArr2HCl i...
Text Solution
|
- Given the equilibrium system NH(4)Cl(s)hArr NH(4)^(+)(aq)+Cl^(-)(aq)...
Text Solution
|
- A catalyst increases the
Text Solution
|
- For the reaction, N(2)+3H(2)hArr2NH(3), the units of K(c) "and" K(p) r...
Text Solution
|
- A 0.20 M solution of methanoic acid has degree of ionization of 0.032....
Text Solution
|
- The equilibrium constant for the reaction N(2)+3H(2)hArr2NH(3) is 70 a...
Text Solution
|
- For the reaction: 4NH(3)(g)+7O(2(g))hArr4NO(2(g))+6H(2)O(g).K(p) is ...
Text Solution
|
- When K(c)gt1 for a chemical reaction,
Text Solution
|
- What will be the effect to increased pressure in the following equilib...
Text Solution
|
- In which reaction will an increase in the volume of the container favo...
Text Solution
|
- Which of the following changes the value of the equilibrium constant ?
Text Solution
|
- Consider the equilibrium reaction: 4NH(3((g)))+3O(2((g)))hArr2N(2((g...
Text Solution
|
- Equilibrium constants K(1) and K(2) for the following equilibria NO(...
Text Solution
|
- A catalyst speeds up a chemical reraction by
Text Solution
|
- For the reaction 2HIhArrH(2)(g)+I(2)(g)
Text Solution
|