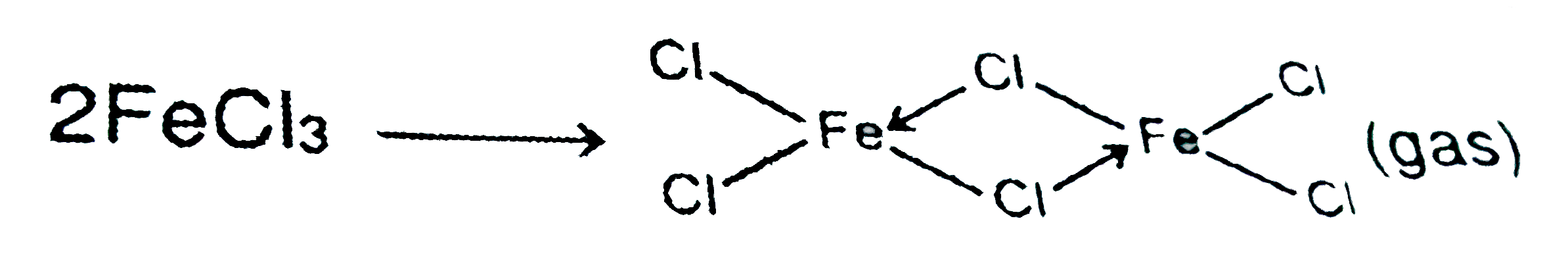A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE ENGLISH|Exercise National Standard Examination In chemistry|35 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE ENGLISH|Exercise High Level Problems|2 VideosD & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS
RESONANCE ENGLISH|Exercise Exercise 3|65 VideosCHEMICAL EQUILIBRIUM
RESONANCE ENGLISH|Exercise Advanced Level Problems (Part-3)(Stage-5)|2 VideosGASEOUS STATE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Hydrocarbon)|18 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-D & F-BLOCK ELEMENTS & THEIR IMPORTANT COMPOUNDS -Additional Problems for Self practice (APSP)
- Which one of the following dissolves in hot concentrated NaOH?
Text Solution
|
- Hot copper wire reacts with oxygen to produce:
Text Solution
|
- A compound is yellow when hot and white when cold. The compound is:
Text Solution
|
- At 300 C FeCl(3):
Text Solution
|
- When FeSO(4) is strongly heated, the number of acidic gases produced i...
Text Solution
|
- On heating ZnCl(2).2H(2)O, the compounds obtained is:
Text Solution
|
- On heating KMnO(4), one among the following is not formed:
Text Solution
|
- Reaction of KMnO(4) in neutral or very weakly alkaline solution can be...
Text Solution
|
- KMnO(4) in excess on treatment with concentrated H(2)SO(4) forms a com...
Text Solution
|
- Which of the following statement is wrong?
Text Solution
|
- The image on an exposed and developed photographic film is due to
Text Solution
|
- The yellow colour solution of Na2CrO4 changes of orange red on passin...
Text Solution
|
- What happens when a molten mixture of K(2)FeO(4) and K(2)CrO(4) is aci...
Text Solution
|
- Why silver chloride is used in photochromic spectacles?
Text Solution
|
- In which of the following reactions, reaction of silver with the given...
Text Solution
|
- Which of the following can be employed for the conversion of potassium...
Text Solution
|
- Reaction of potassium chromate and CuSO(4) in aqueous solution produce...
Text Solution
|
- Predict the product formed and its colour: When MnO2 is fused with KO...
Text Solution
|
- Transuranic elements begin with
Text Solution
|
- Lanthanide contraction is observed in:
Text Solution
|