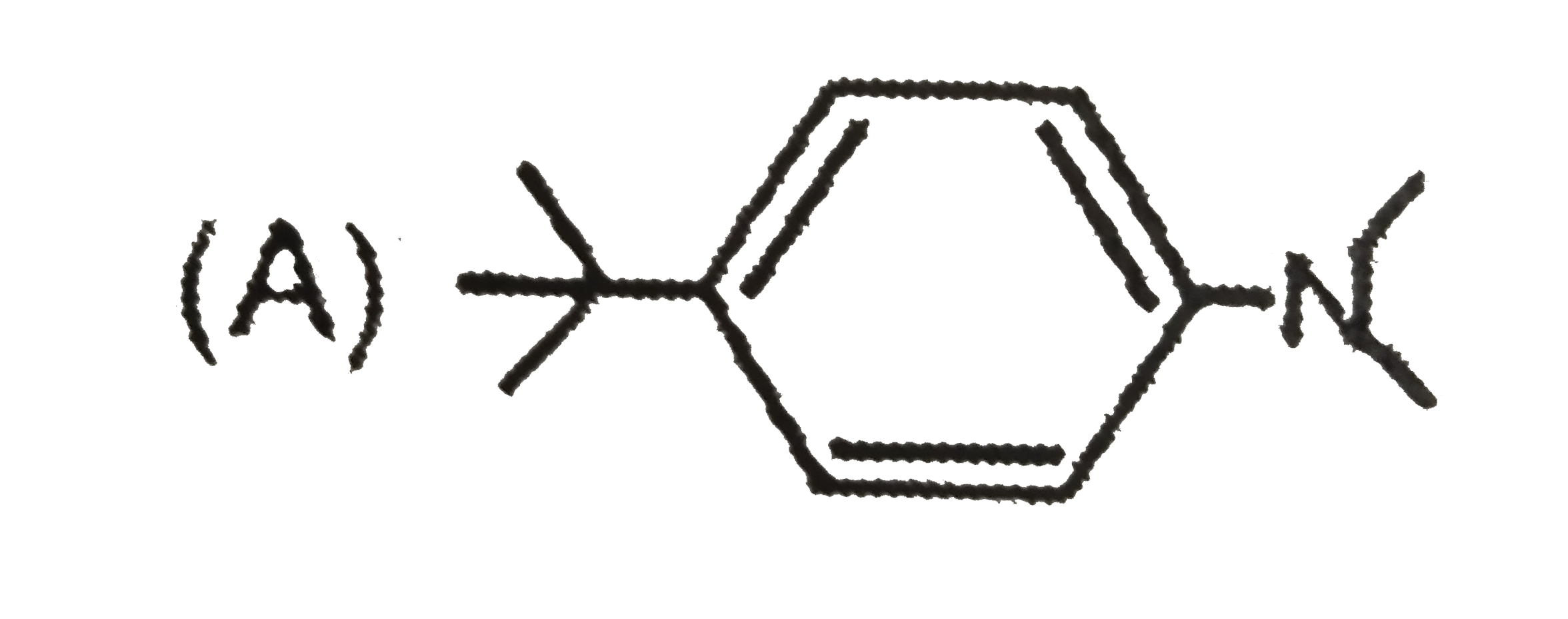
A
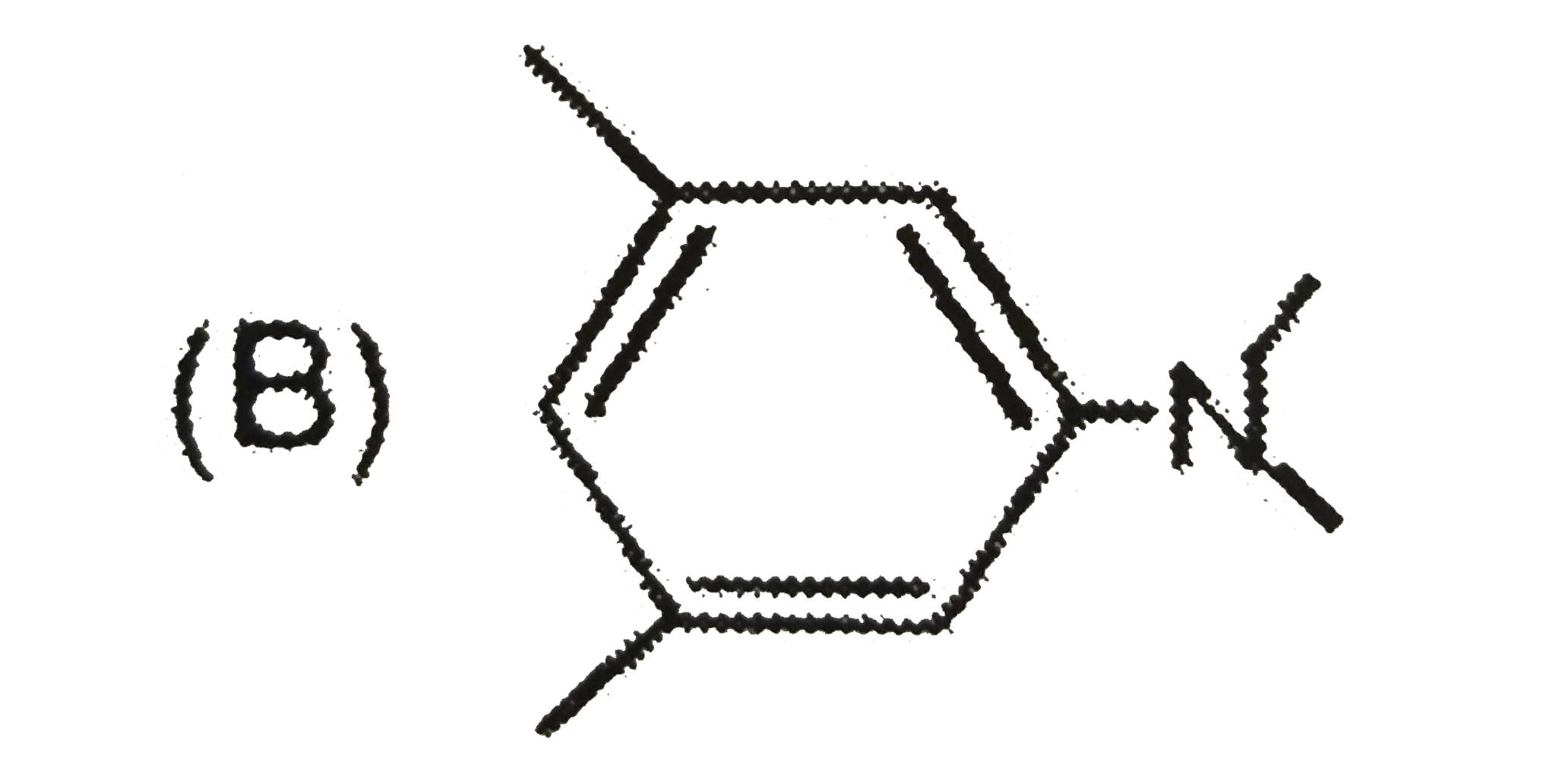
B
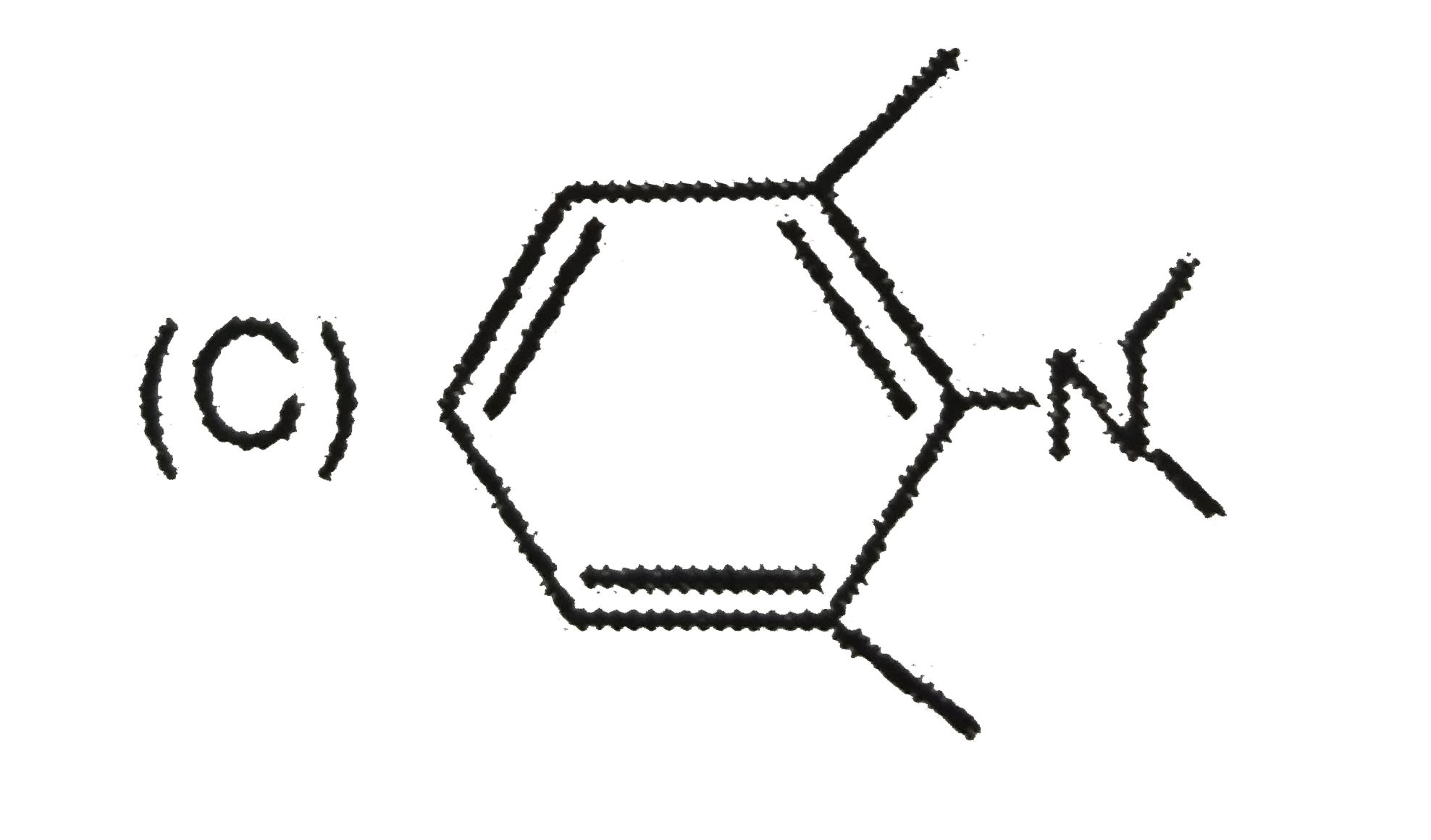
C
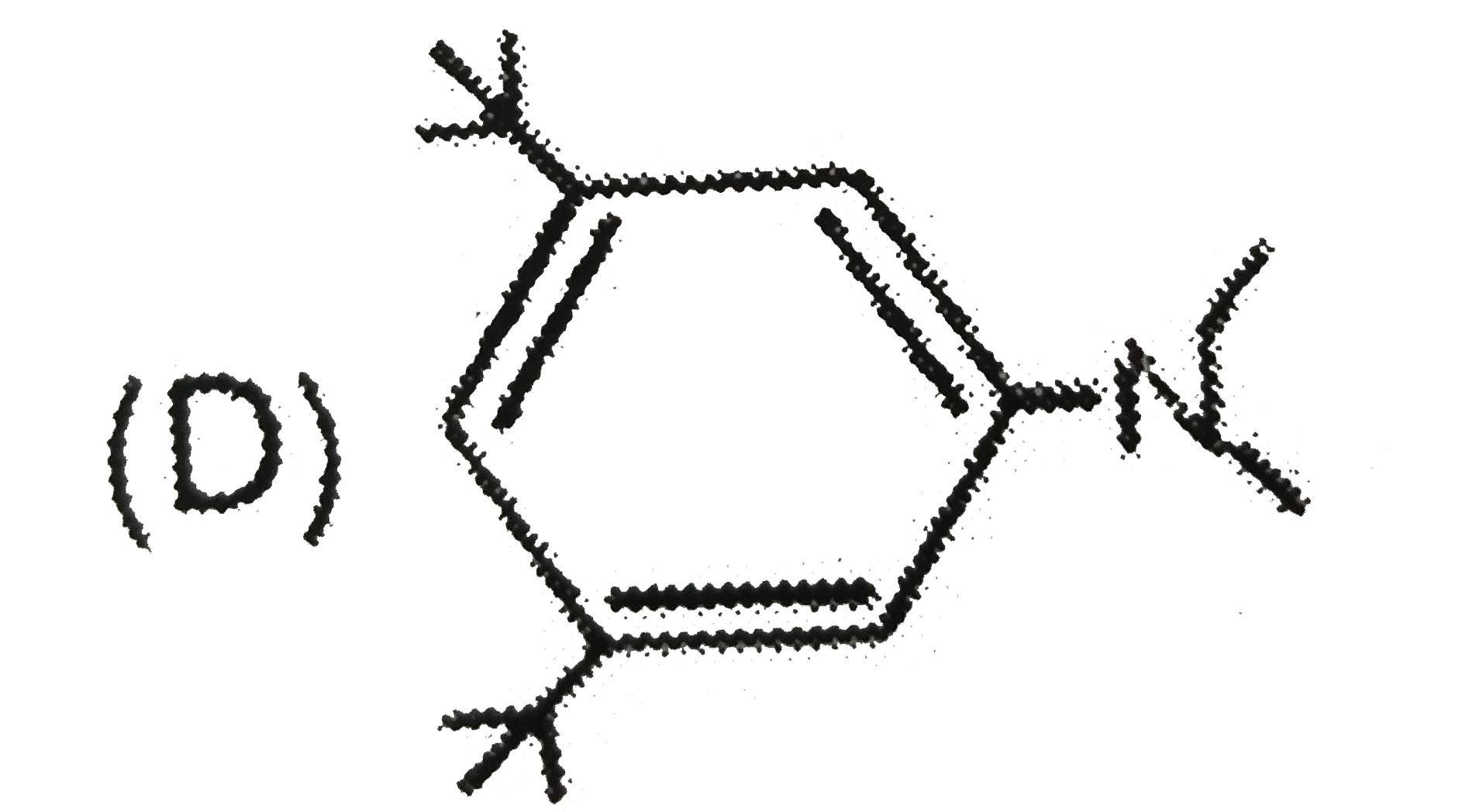
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise EXERCISE-1 PART-III (MATCH THE COLUMN)|1 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise EXERCISE-2 PART-I|20 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise PART-III : PRACTICE TEST-19|1 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-III: Section-5: Matching List Type|1 VideosHYDROGEN AND ITS COMPOUNDS
RESONANCE ENGLISH|Exercise INORGANIC CHEMISTRY(Hydrogen & its compunds Y environment chemistry)|33 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GENERAL ORGANIC CHEMISTRY-I-EXERCISE-1 PART-II
- Which of the following group show +M and -l effect?
Text Solution
|
- Which of the following group shown +mgt - I effect?
Text Solution
|
- Which of the following group show -m and -I effect?
Text Solution
|
- +I effect is shown by
Text Solution
|
- Weakest acid is:
Text Solution
|
- Maximum extent of steric inhibition of resonance can be expected in
Text Solution
|
- Hyperconjugation involves overlap of which of the following orbitals?
Text Solution
|
- Which of the following cannot exhibit hypercojugation -
Text Solution
|
- Which of the following will give maximum number of isomers?
Text Solution
|
- Which one of the following has inductive, mesomeric and hyperconjugati...
Text Solution
|
- Which of the following group has the maximum hyperconjugation effect ?
Text Solution
|
- Which of the following is aromatic hydrocarbon ?
Text Solution
|
- Identify the aromatic amino acid from the given option.
Text Solution
|
- Alkanes burn with a smoky flame.
Text Solution
|
- The decreasing order of electron density on the ring is :
Text Solution
|
- Correct dipole moment order is
Text Solution
|
- Arrange following compounds in decreasing order of their dipole moment...
Text Solution
|
- The stability order of alkene in following compounds is :
Text Solution
|
- Select the correct statement about this compound.
Text Solution
|
- Arrange the satbility of following :
Text Solution
|