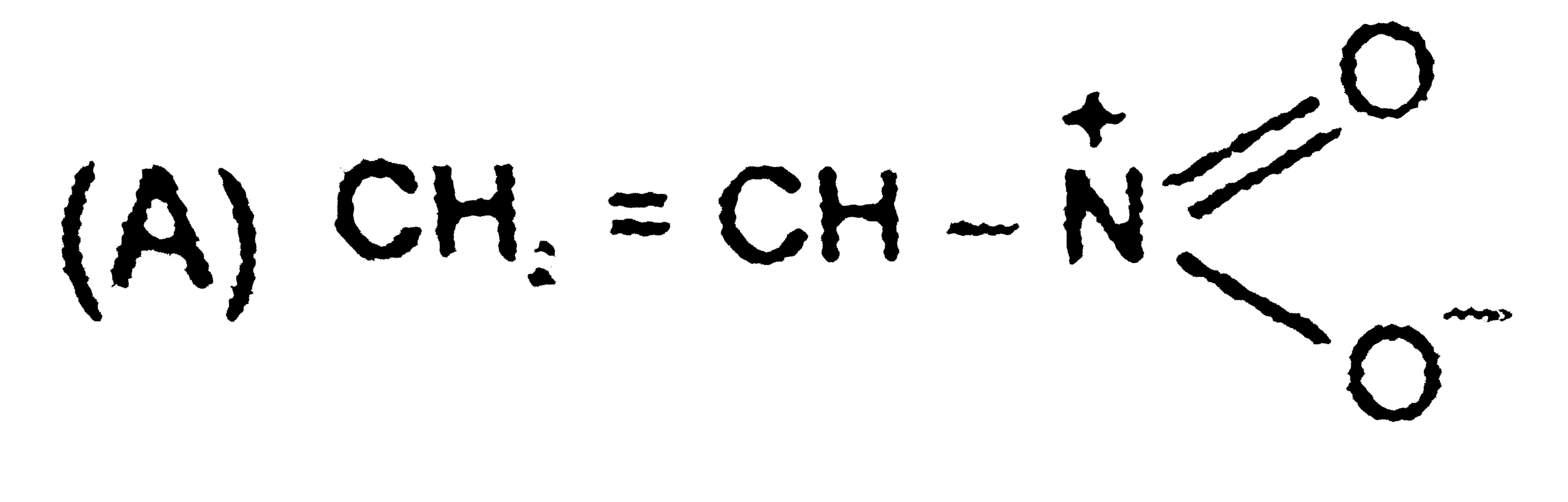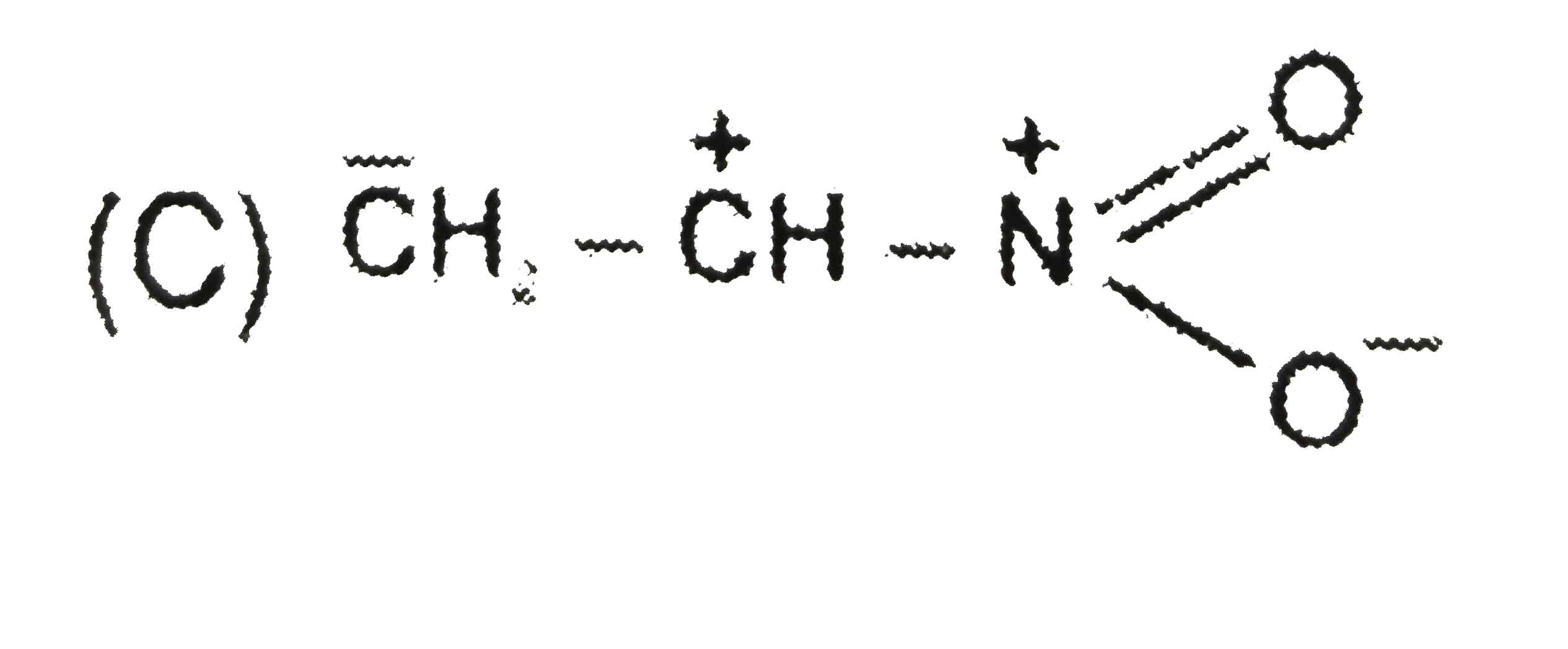A
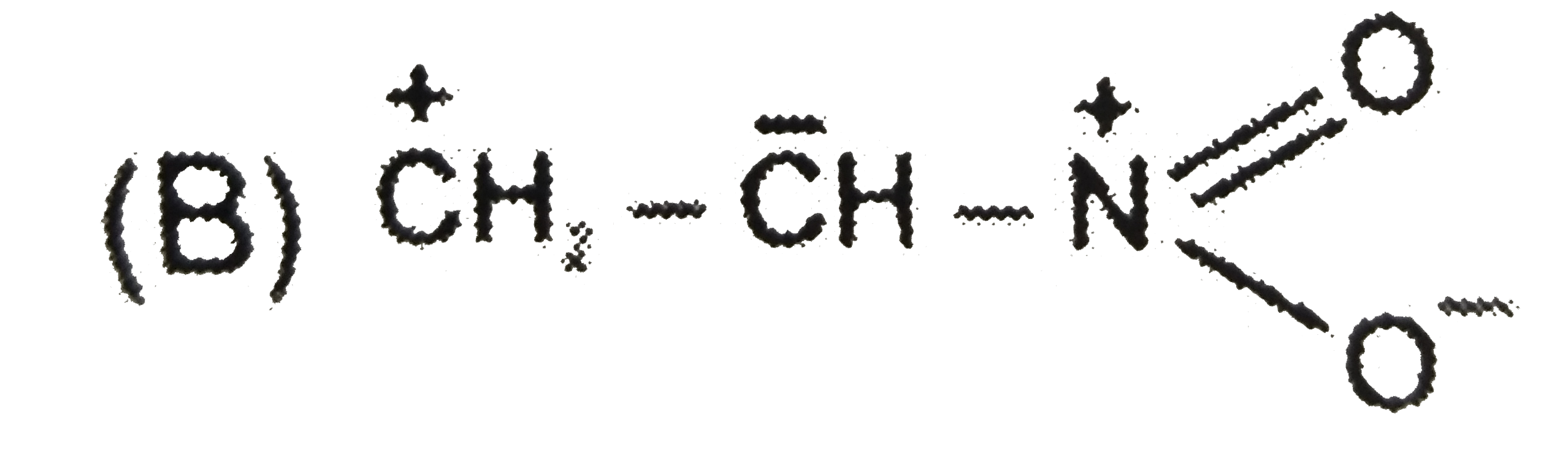
B
C
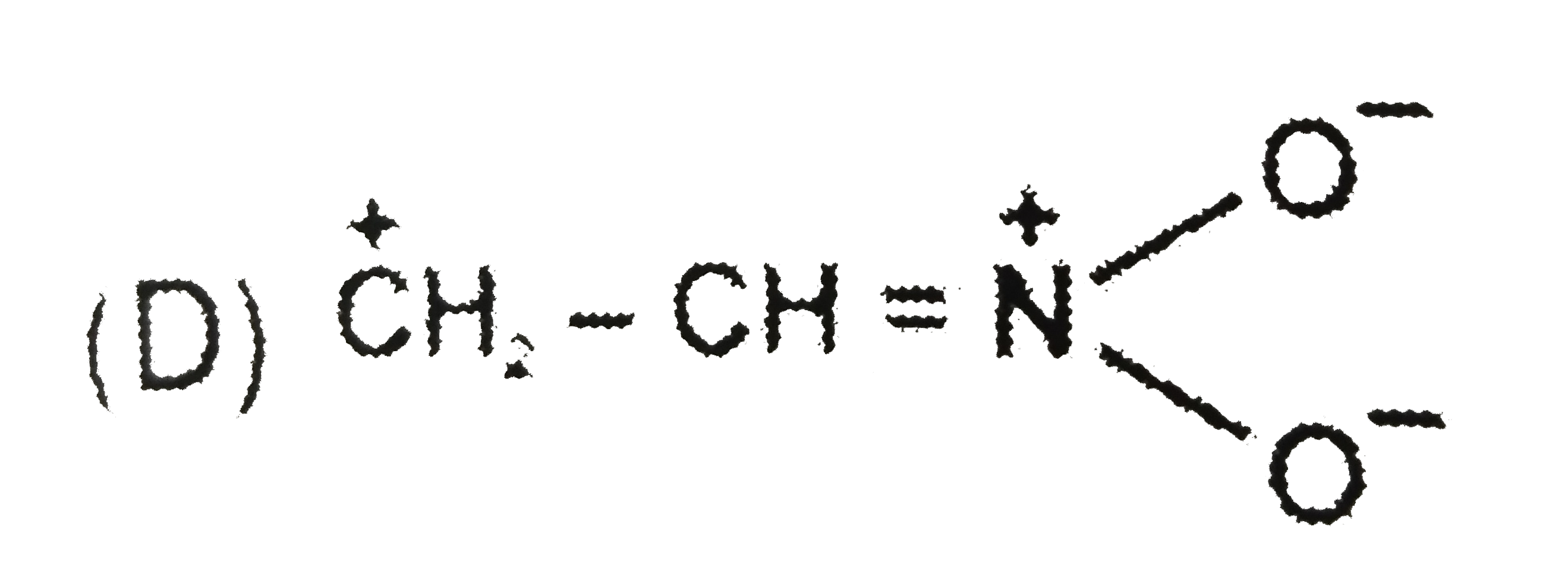
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise EXERCISE-2 PART-II SINGLE AND DOUBLE VALUE INTEGER TYPE|11 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise EXERCISE-2 PART-III : ONE OR MORE THAN ONE OPTIONS CORRECT TYPE|9 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise EXERCISE-1 PART-III (MATCH THE COLUMN)|1 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-III: Section-5: Matching List Type|1 VideosHYDROGEN AND ITS COMPOUNDS
RESONANCE ENGLISH|Exercise INORGANIC CHEMISTRY(Hydrogen & its compunds Y environment chemistry)|33 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GENERAL ORGANIC CHEMISTRY-I-EXERCISE-2 PART-I
- The most unlikely representation of resonance structures of p-nitrophe...
Text Solution
|
- In which of the following carbocations, delocalisation of positive cha...
Text Solution
|
- Decreasing order of potential energy of the following cations is :
Text Solution
|
- Stability order of the following species ?
Text Solution
|
- In which of the following element +1 oxidation state is more stable :
Text Solution
|
- Which of the following is correct order of stability:
Text Solution
|
- Least contributing resonating structure of nitroethene is :
Text Solution
|
- Which of the following statement is correct ?
Text Solution
|
- The decreasing order of bond length of C=C bond in the following compo...
Text Solution
|
- Which of the following is correct about the following compound
Text Solution
|
- The correct order of +M effect of 'N' containing functional group on b...
Text Solution
|
- In which case the sigma bond pair and pi bond pair of electrons both a...
Text Solution
|
- The correct stability order of given resonating structures
Text Solution
|
- The longest C-N bond length in the given compound is :
Text Solution
|
- Select the correct order of heat of hydrogenation?
Text Solution
|
- H(3)C-overset(o+)CH-CH=CH(2) does not involve :
Text Solution
|
- Stability of pi-bond in following alkenes in the increasing order is :...
Text Solution
|
- In this molecules, pi-electron density is more on :
Text Solution
|
- If the given compound is planar. Select the correct statement.
Text Solution
|
- The correct order of electron density in aromatic ring of following co...
Text Solution
|