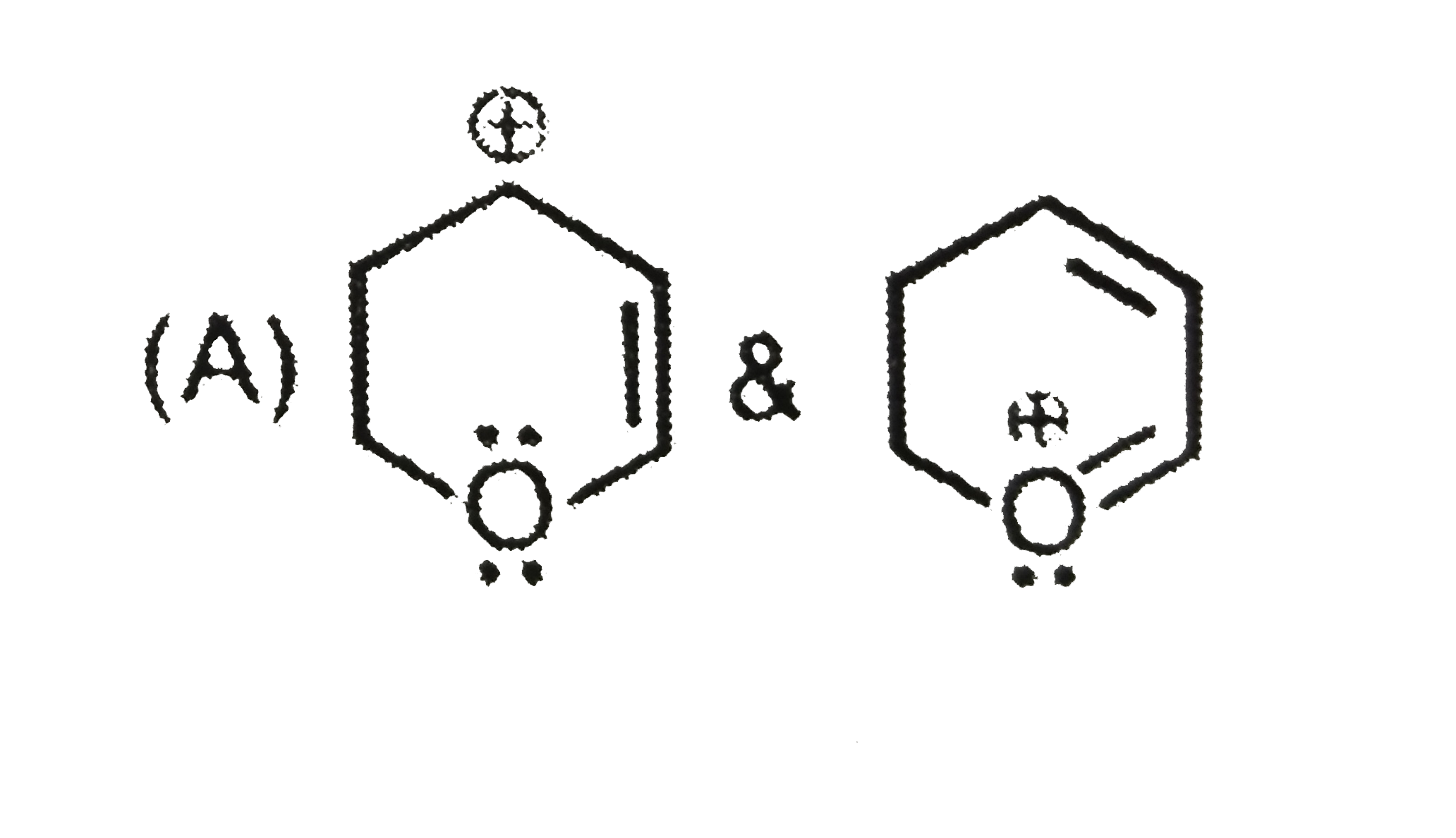
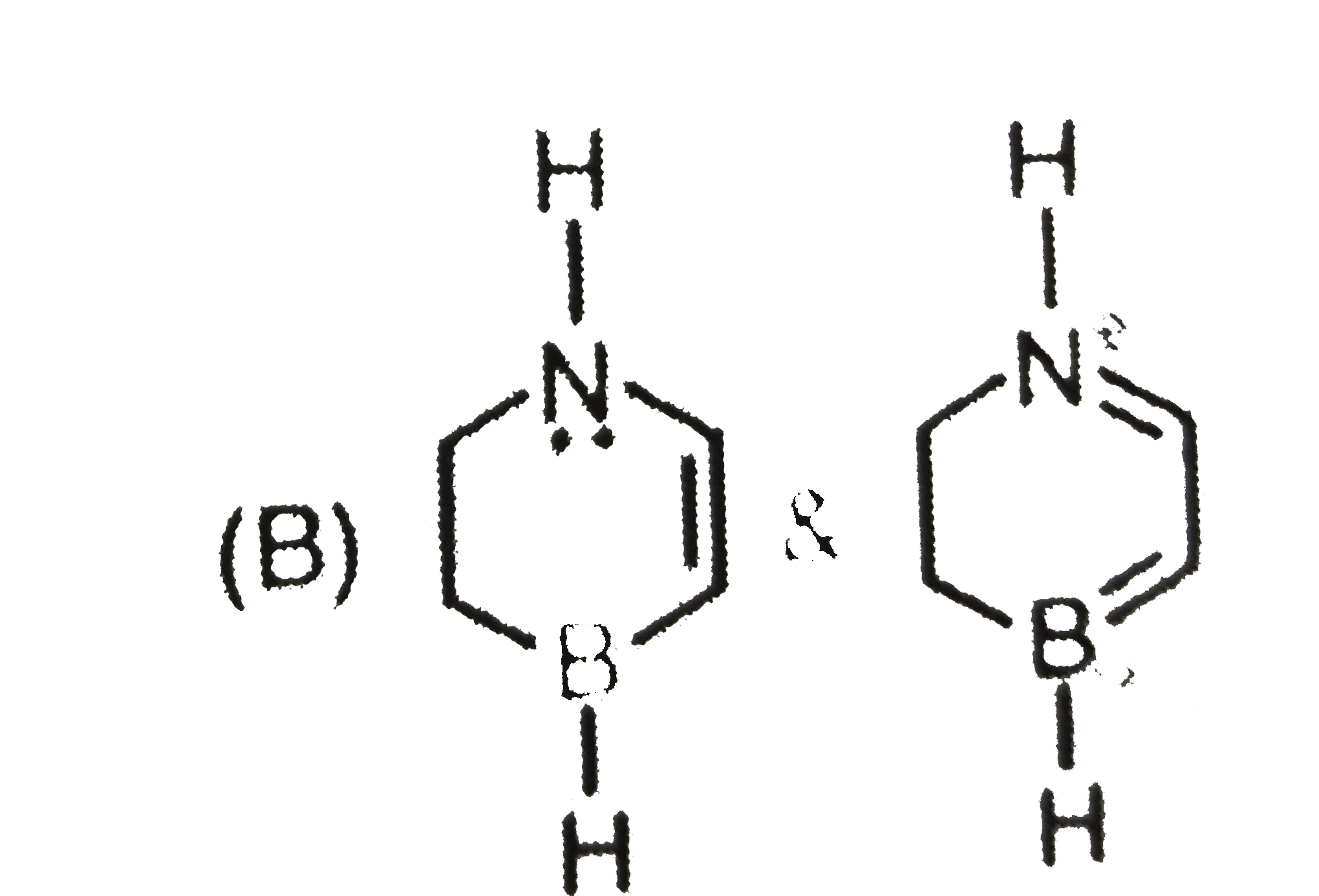
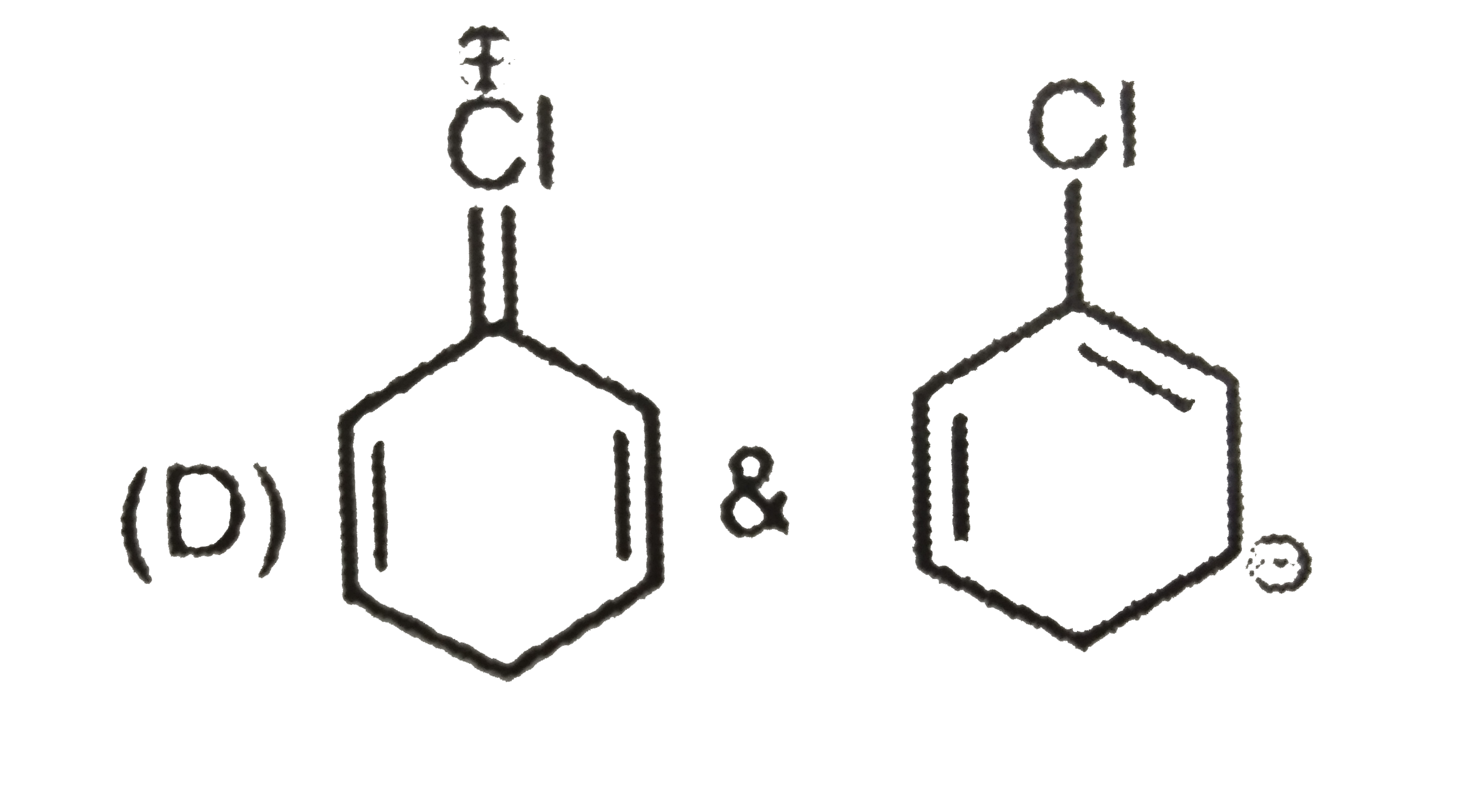
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise EXERCISE-2 PART-IV : COMPREHENSION|5 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise EXERCISE-3|11 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise EXERCISE-2 PART-II SINGLE AND DOUBLE VALUE INTEGER TYPE|11 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-III: Section-5: Matching List Type|1 VideosHYDROGEN AND ITS COMPOUNDS
RESONANCE ENGLISH|Exercise INORGANIC CHEMISTRY(Hydrogen & its compunds Y environment chemistry)|33 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GENERAL ORGANIC CHEMISTRY-I-EXERCISE-2 PART-III : ONE OR MORE THAN ONE OPTIONS CORRECT TYPE
- Which statement is/are true about resonance ?
Text Solution
|
- Which of the following statement is incorrect about resonance?
Text Solution
|
- In which of the following pairs of compounds, will second structure ha...
Text Solution
|
- Which of the following pairs represents resonating structures ?
Text Solution
|
- In which of the following compounds delocalisation of electrons and sh...
Text Solution
|
- Explain resonance in benzene.
Text Solution
|
- Which of the following is/are correct :
Text Solution
|
- Which of the following is/are correct statement :
Text Solution
|
- Which is the correct order of bond length ?
Text Solution
|