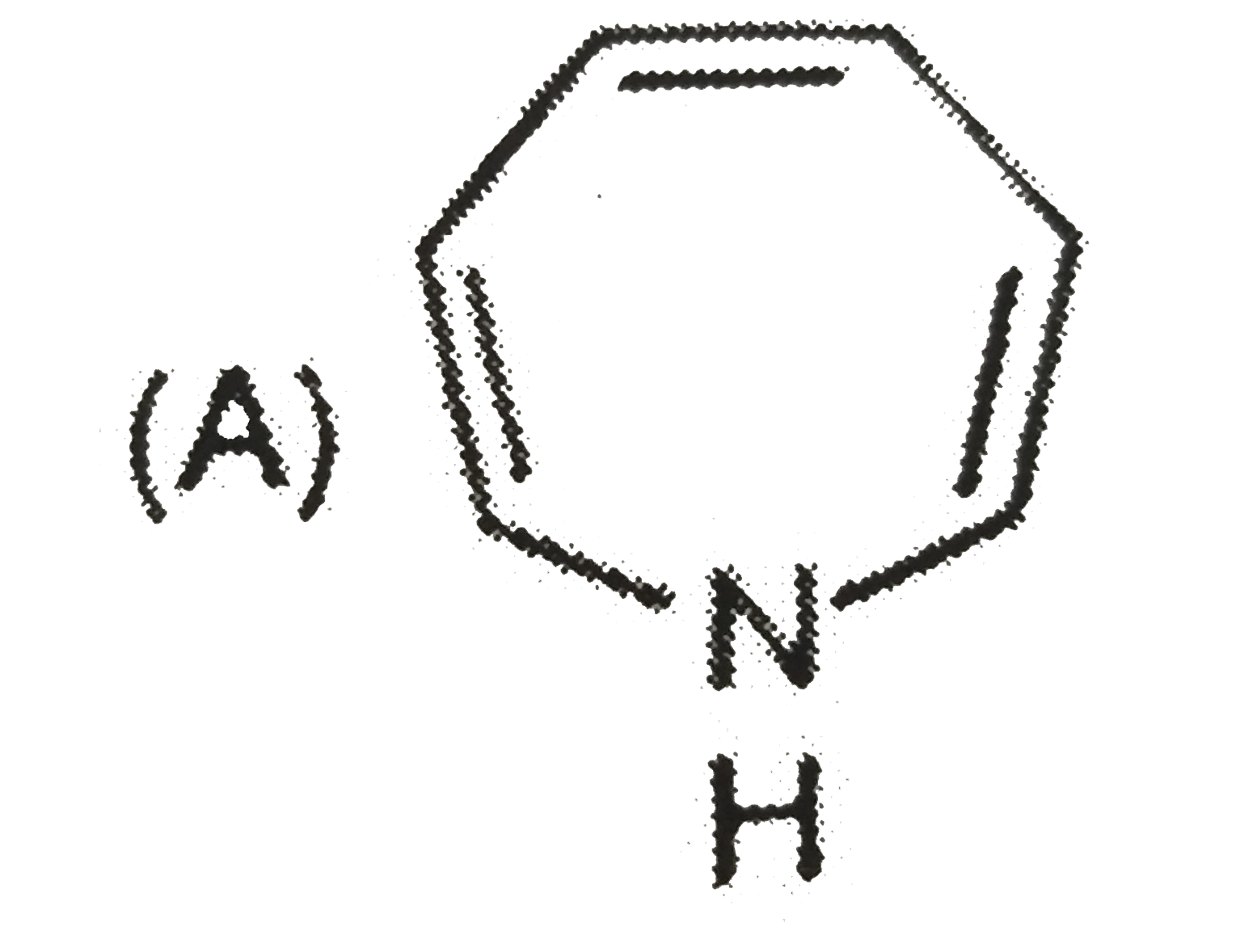
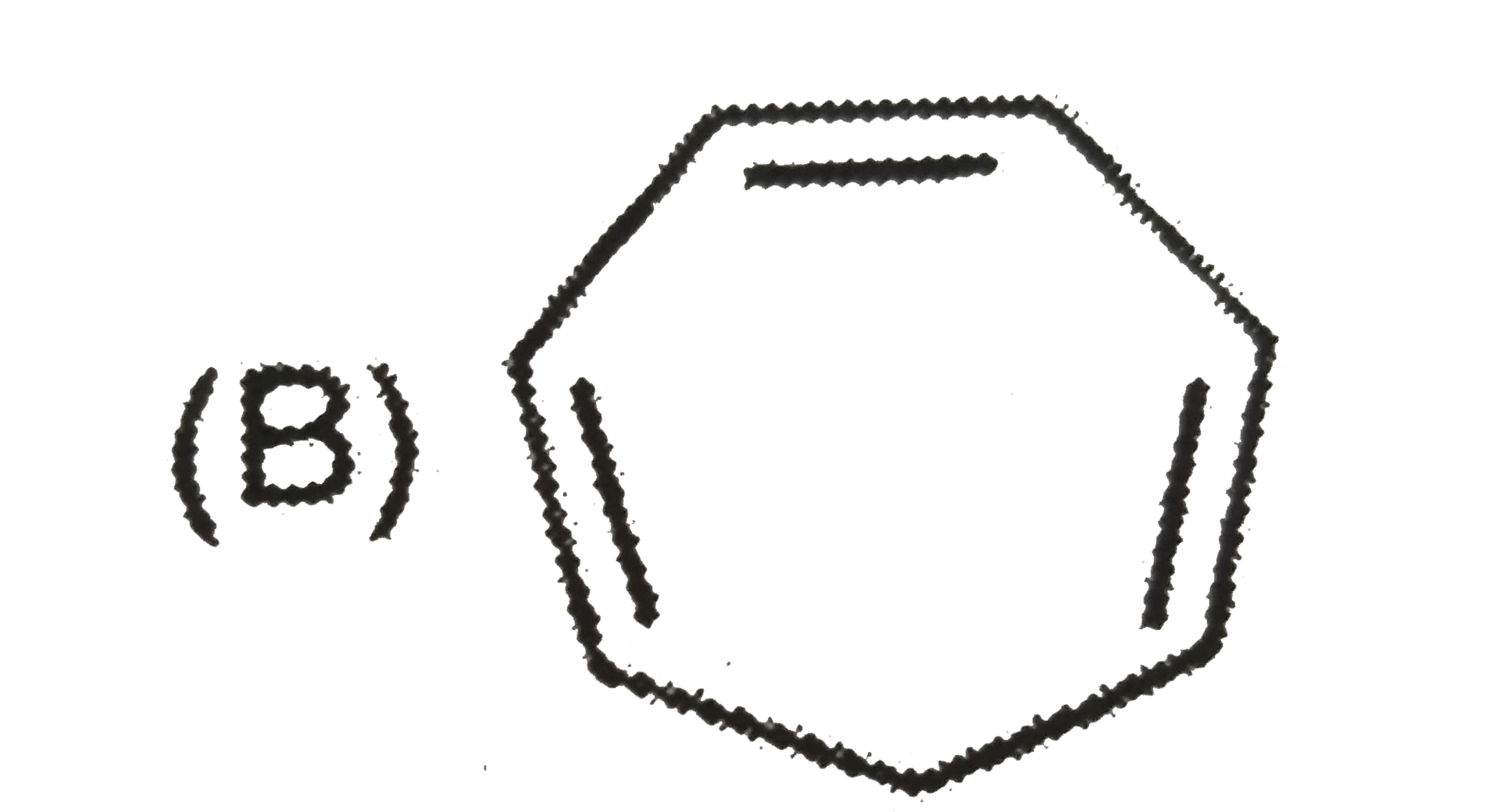
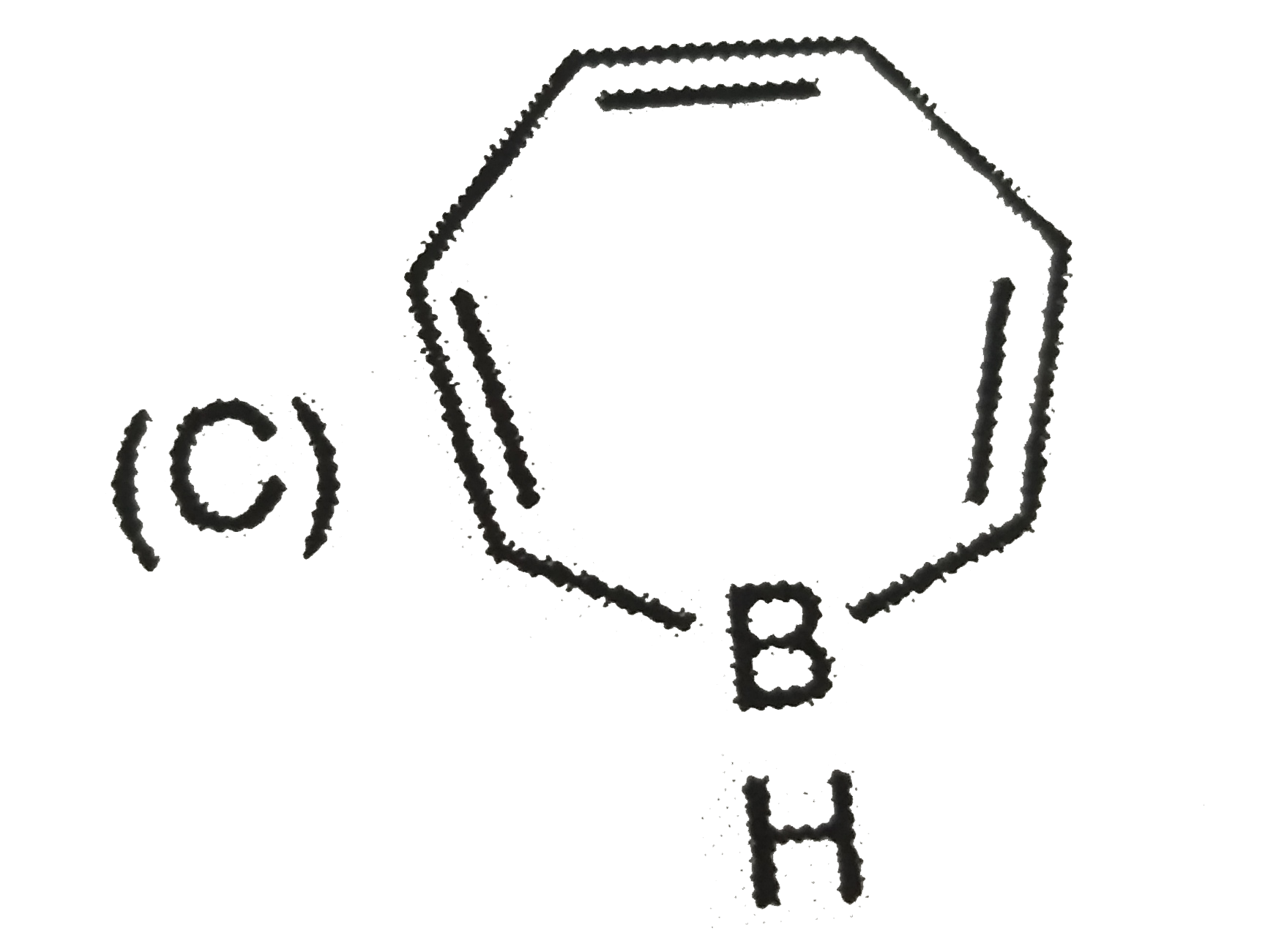
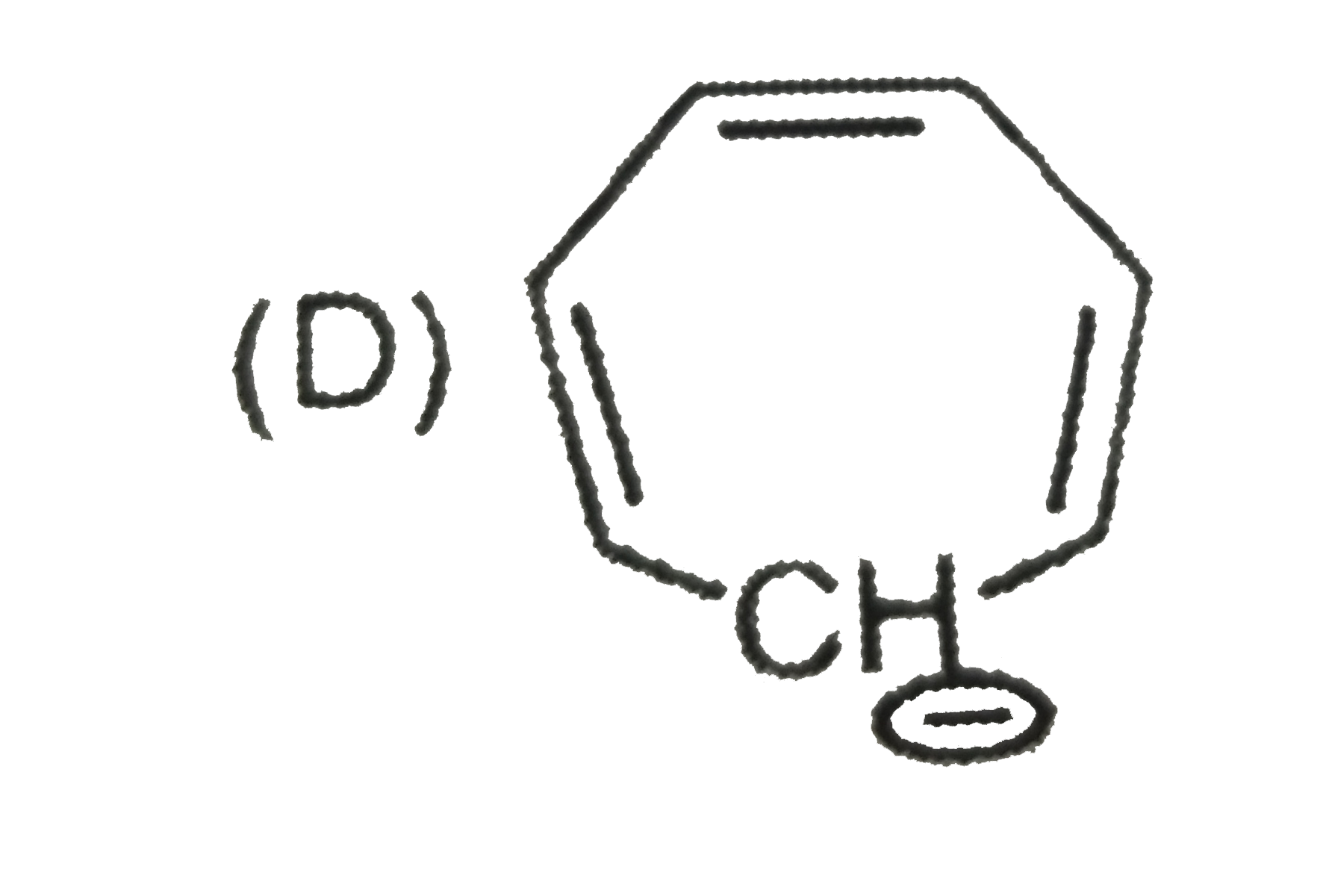
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise PART-III : PRACTICE TEST-2|1 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise PART-III : PRACTICE TEST-3|1 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise PRACTICE TEST-1|30 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-III: Section-5: Matching List Type|1 VideosHYDROGEN AND ITS COMPOUNDS
RESONANCE ENGLISH|Exercise INORGANIC CHEMISTRY(Hydrogen & its compunds Y environment chemistry)|33 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GENERAL ORGANIC CHEMISTRY-I-PART-2(NSEC)
- Select the most stable carbonium ion from amongst the following
Text Solution
|
- Which of the following pairs represents resonating structures ?
Text Solution
|
- The aromatic compound would be
Text Solution
|
- Inductive effect is a polarisation of a
Text Solution
|
- Match the resonance energies 67, 88 and 121 kJ "mol"^(-1) for the foll...
Text Solution
|
- The pair of resonanating structures among the following is
Text Solution
|
- Identity the aromatic compound from the following.
Text Solution
|
- Which of the following species is aromatic?
Text Solution
|
- The number of pi electrons required for a planar cyclic conjugated sys...
Text Solution
|
- Following is an example of CH(2)=CH-overset(O)overset(||)CHharrover...
Text Solution
|
- The relative stabilites of the following carbocations is : H(2)"CO C...
Text Solution
|
- Identify the odd species out (which of the species among the following...
Text Solution
|
- Which of the following represents the true order of bond dissociation ...
Text Solution
|
- The correct order of dipole moment for the following molecules is
Text Solution
|
- The order of decreasing stability is :
Text Solution
|
- The most Carbocations, carbanions, free radicals and radical cation ar...
Text Solution
|
- The electronegativities of acetylene, ethylene and ethane are in the o...
Text Solution
|
- Which of the following structure is aromatic ?
Text Solution
|
- Which of the following is most stable?
Text Solution
|
- How many hperconjugative structures are possible in the following carb...
Text Solution
|