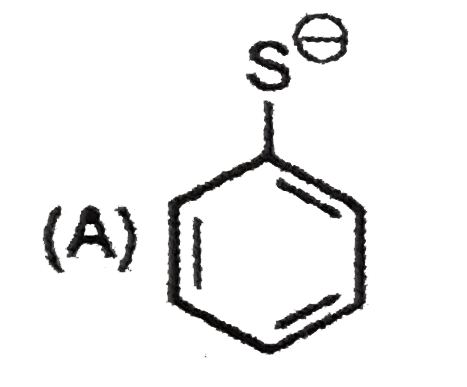
A
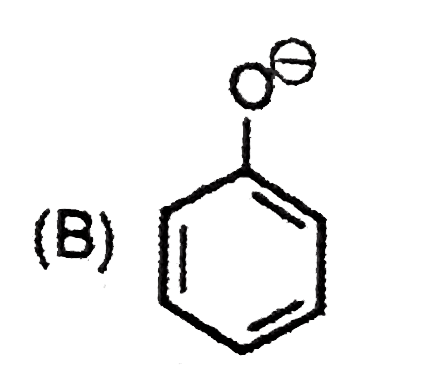
B
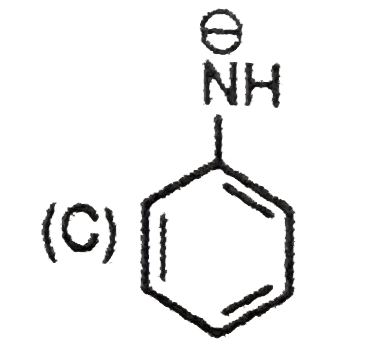
C
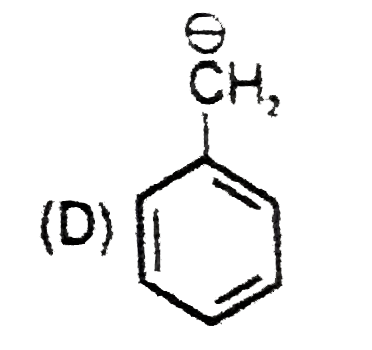
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
GENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-III: Match the Column|2 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Part-I : Only One Option Correct Type|22 VideosGENERAL ORGANIC CHEMISTRY II
RESONANCE ENGLISH|Exercise Exercise-1|20 VideosGASEOUS STATE
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Hydrocarbon)|18 VideosGENERAL ORGANIC CHEMISTRY-I
RESONANCE ENGLISH|Exercise PART-III : PRACTICE TEST-19|1 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-GENERAL ORGANIC CHEMISTRY II-Part-II Only One Option Correct Type
- Which of the following is least stable carbanion ?
Text Solution
|
- The most stable anion is:
Text Solution
|
- In which of the following pairs of carbarion the first one is more sta...
Text Solution
|
- Arrange the followinng carbocations in decreasing order of stability
Text Solution
|
- The most stable ion is .
Text Solution
|
- Arrange the following carbanions in increasing order of stability:
Text Solution
|
- Among the following, the paramagnetic species is:
Text Solution
|
- The stability of given free radicals in decreasing order is (i) CH(3...
Text Solution
|
- Which of the following is the correct order of stability of free radic...
Text Solution
|
- Most stable radical among the following is :
Text Solution
|
- Arrange the following radicals in decreasing order of their stability.
Text Solution
|
- Least stable radical among the following is :
Text Solution
|
- The most stable carbocation is
Text Solution
|
- The most stable carbocation is:
Text Solution
|
- Which of the following shows the correct order of decreasing stability...
Text Solution
|
- Which of the following is the arranged more stable carbocation of the ...
Text Solution
|
- Most stable rearranged form of given carbocations is:
Text Solution
|
- Which of the following in the rearranged in the rearranged more stable...
Text Solution
|
- The correct basic strength order of following anions is:
Text Solution
|
- Which of the following shows the correct order of decreasing basicity ...
Text Solution
|