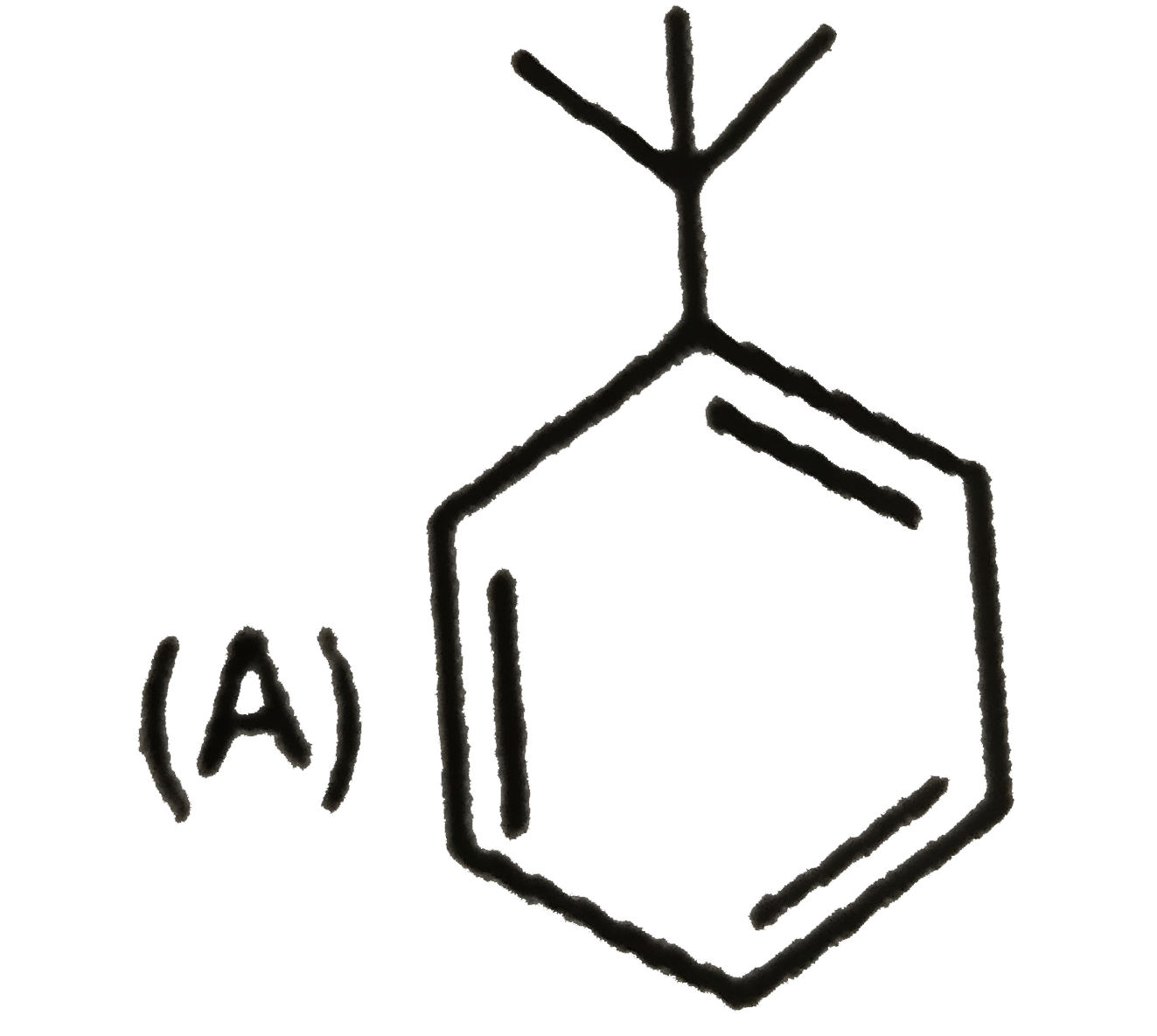
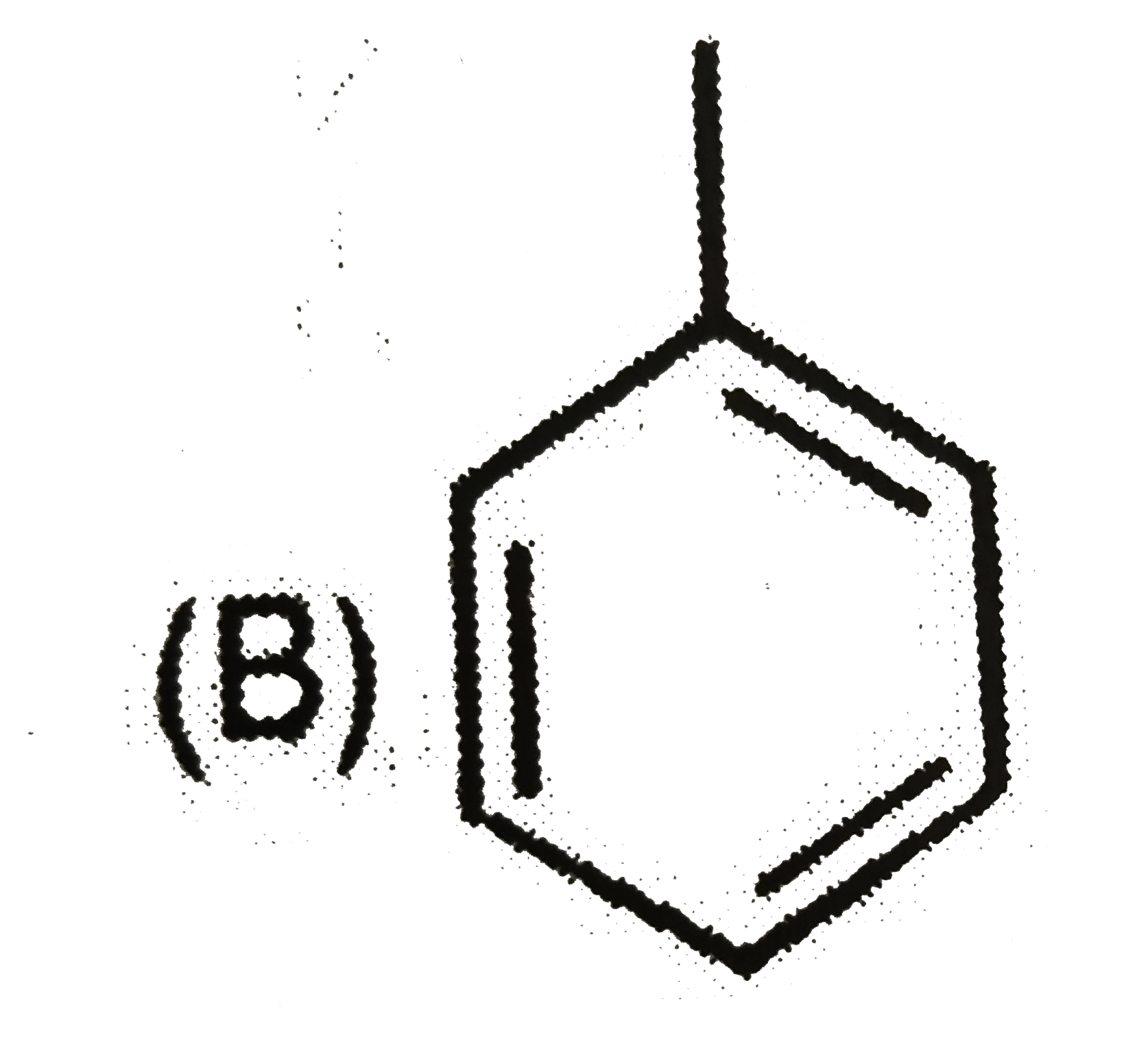
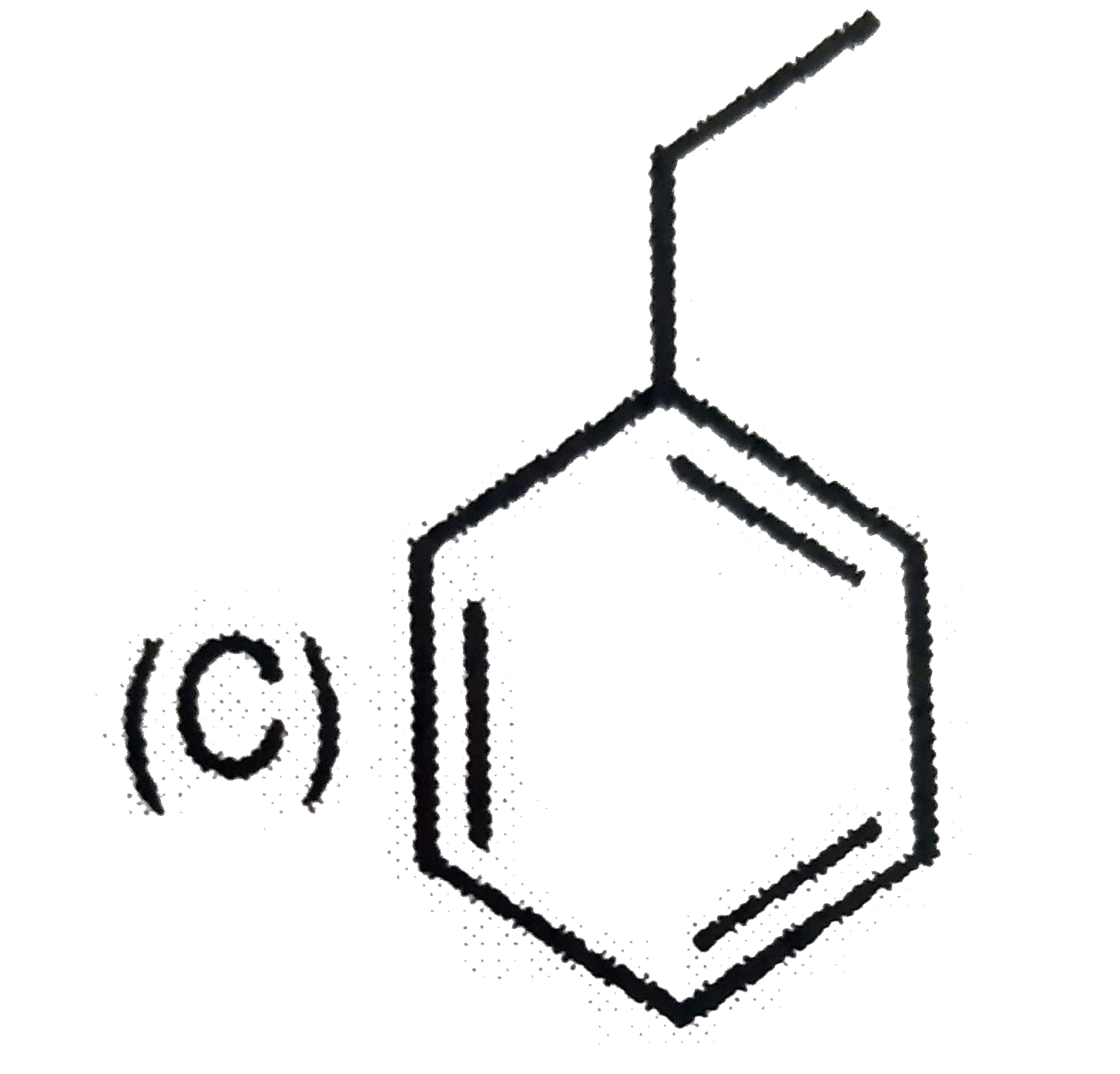
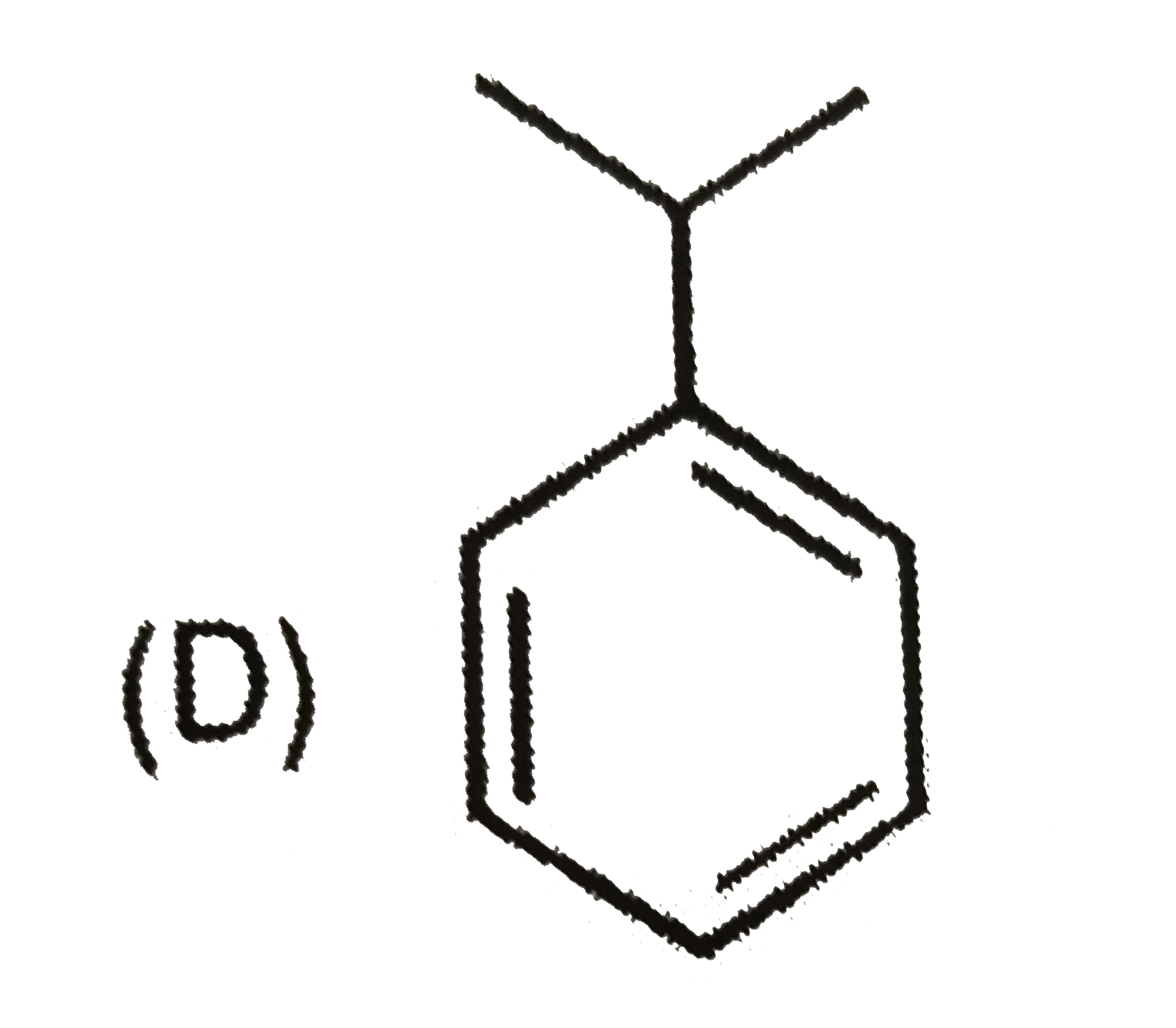
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise Exercise-1 Part-3|2 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise Exercise-2 Part-1|11 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise APSP Part - 3|22 VideosMOLE CONCEPT
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS - II-Exercise-1 Part-2
- The reagent used for Friedel-Craft's reaction is :
Text Solution
|
- Which of the following will undergo sulphonation at fastest rate ?
Text Solution
|
- Which among the following is deactivating group ?
Text Solution
|
- The compound X in the reaction is :
Text Solution
|
- Toluene o/p orienting with respect to an electrophilic substitution re...
Text Solution
|
- Which of the following structures correspond to the product expected, ...
Text Solution
|
- Benzene undergoes substituion reaction more easily than addition becau...
Text Solution
|
- Which one is o, p-directiong group for electrophliic substitution reac...
Text Solution
|
- In the free-radical chlorination of methane, the chain-intiation step ...
Text Solution
|
- The maximum ease of abstraction of a hydrogen atom by a chlorine atom ...
Text Solution
|
- Methane reacts with excess of chlorine is diffused sunlight to give th...
Text Solution
|
- The above reaction is known as
Text Solution
|
- In which of the following pairs the bromination of first member is eas...
Text Solution
|
Text Solution
|
- CH3-undersetunderset(CH3)(|)C=CH2+HBr overset(R2O2)to Product is :
Text Solution
|
- One of the following which does not observe the anti-Markovnikoff's ad...
Text Solution
|
- What are hybridisation states of each carbon atom in the following com...
Text Solution
|
- Arrange in decreasing order of reactivity with HCl :
Text Solution
|
Text Solution
|
- In the given reaction CH(3)-CH=CH-CH(2)-OHoverset(PC C)(to) Product ...
Text Solution
|