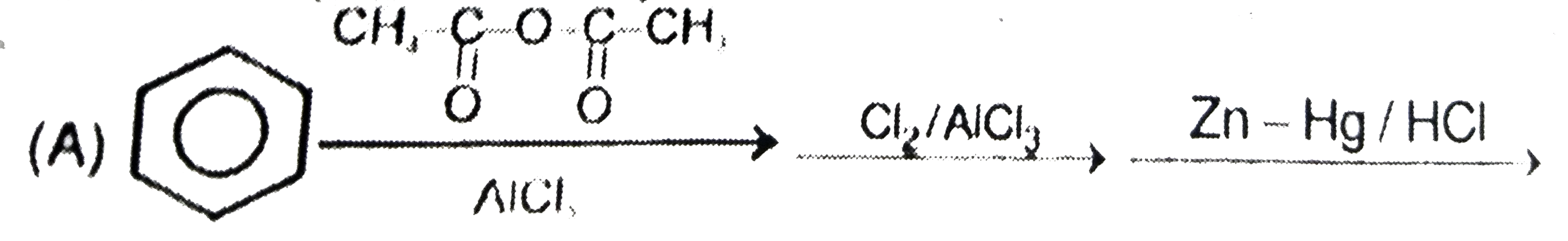
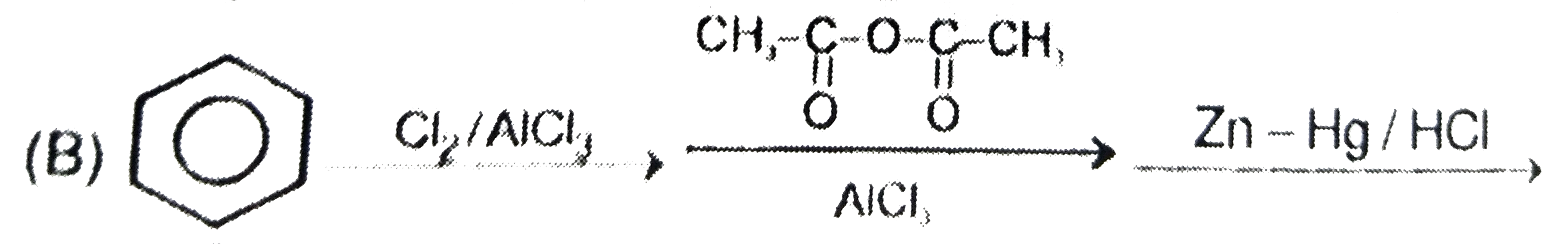
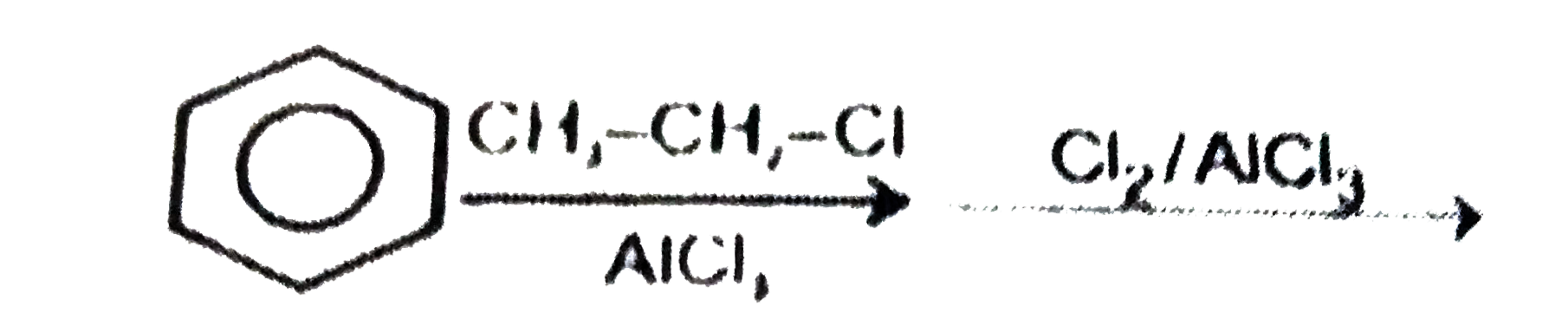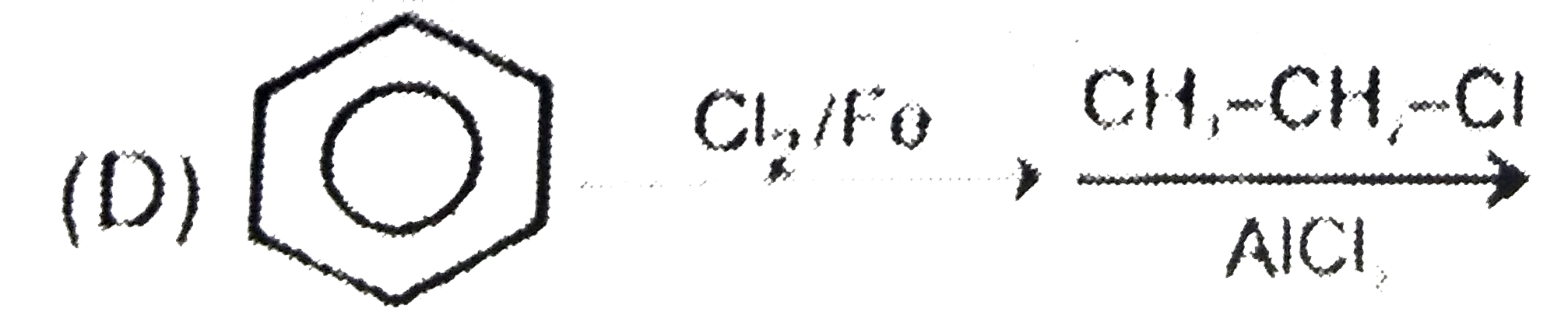A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise Exercise-2 Part-2|8 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise Exercise-2 Part-3|10 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise Exercise-1 Part-3|2 VideosMOLE CONCEPT
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS - II-Exercise-2 Part-1
- Which of the following species is expected to have maximum enthalpy in...
Text Solution
|
- Which step is used to produce 1-Chloro-3-ethylbenzene
Text Solution
|
Text Solution
|
- Which statement is correct about photochemical bromination of Butane ?...
Text Solution
|
- Identify the incorrect statement // statement : (i) Alkynes are more...
Text Solution
|
- The correct order of reactivity towards electrophilic substitution is
Text Solution
|
Text Solution
|
Text Solution
|
- The reaction of one equivalent of HBr with CH2=CH-CH2-C-=CH gives :
Text Solution
|
- The reaction of one equivalent of HBr with CH(2)=CH-C-=CH gives
Text Solution
|
- At given temperature , these reaction tell about control of reaction w...
Text Solution
|