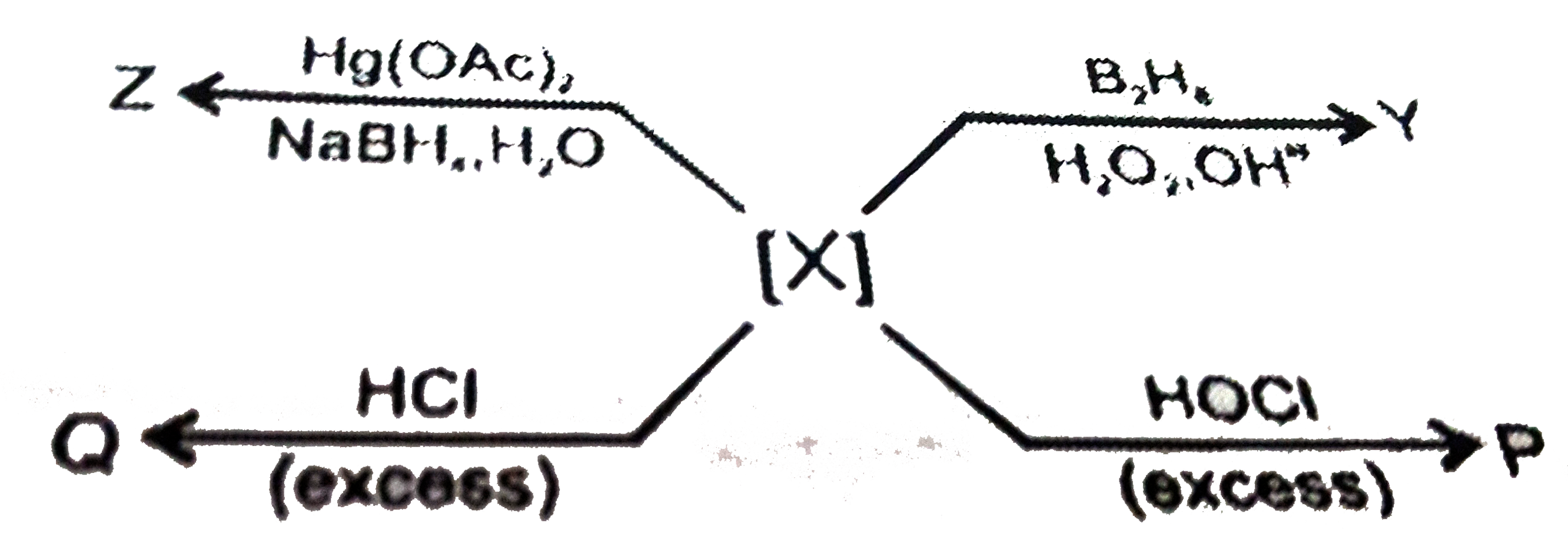
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise Exercise-3 Part-1|20 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise Exercise-3 Part-2|34 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise Exercise-2 Part-3|10 VideosMOLE CONCEPT
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS - II-Exercise-2 Part-4
- Three acyclic alkenes (x,y,z) on catalytic hyrogenation give same alka...
Text Solution
|
- A Hydrocarbon X (M.F.C4H6) produces an aldehyde Y through Hydroboratio...
Text Solution
|
- A Hydrocarbon X (M.F.C4H6) produces an aldehyde Y through Hydroboratio...
Text Solution
|
- Ketone is formed by the reaction
Text Solution
|
- Which of the following is non-correct for substitution reaction.
Text Solution
|