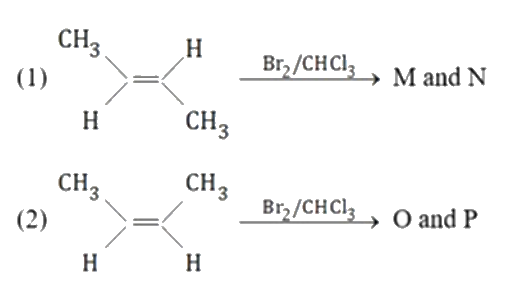
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise Exercise-3 Part-2|34 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise APSP Part - 1|30 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise Exercise-2 Part-4|5 VideosMOLE CONCEPT
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS - II-Exercise-3 Part-1
- In the presence of peroxide, hydrogen chloride and hydrogen iodide do ...
Text Solution
|
- The reaction of propene with HOCl proceeds via the addition of :
Text Solution
|
- Assertion : Addition of bromine to trans-but-2-ene yields meso-2,3-di...
Text Solution
|
- Identify the correct order of reactivity in electrophilic substitution...
Text Solution
|
- Consider the following reaction CH(3)-underset(D)underset(CH)-unders...
Text Solution
|
- Ph - C -= C-CH(3) overset(Hg^(2+)//H(2)SO(4)(aq)) to A The major pro...
Text Solution
|
- How many chiral compounds are possible on monochlorination of 2-methyl...
Text Solution
|
- The product of acid catalyzed hydration of 2-phenylpropene is :
Text Solution
|
- (CH3)2CH-CH2CH3overset(Cl2//h upsilon)to[N]underset("distillation")ove...
Text Solution
|
- 2-propanol will be product of which one of the following reactions? Mu...
Text Solution
|
- In the following reaction, the structure of the major product 'X'...
Text Solution
|
- Statement 1: In bromobenzene upon reaction with Br(2)//Fe gives ,4 dib...
Text Solution
|
- The compounds P,Q and S were separately to nitration using HNO3//H2SO4...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- The maximum number of isomers (including stereoisomers ) that are poss...
Text Solution
|
- Different possible thermal decomposition pathways for peroxyesters are...
Text Solution
|
- In the following reaction, the major product is
Text Solution
|
- For the following compound during monobromination reaction, the number...
Text Solution
|
- Among the following reaction(s), which gives(give) tert-butyl benzene ...
Text Solution
|
- The correct statement for the following addition reaction is
Text Solution
|