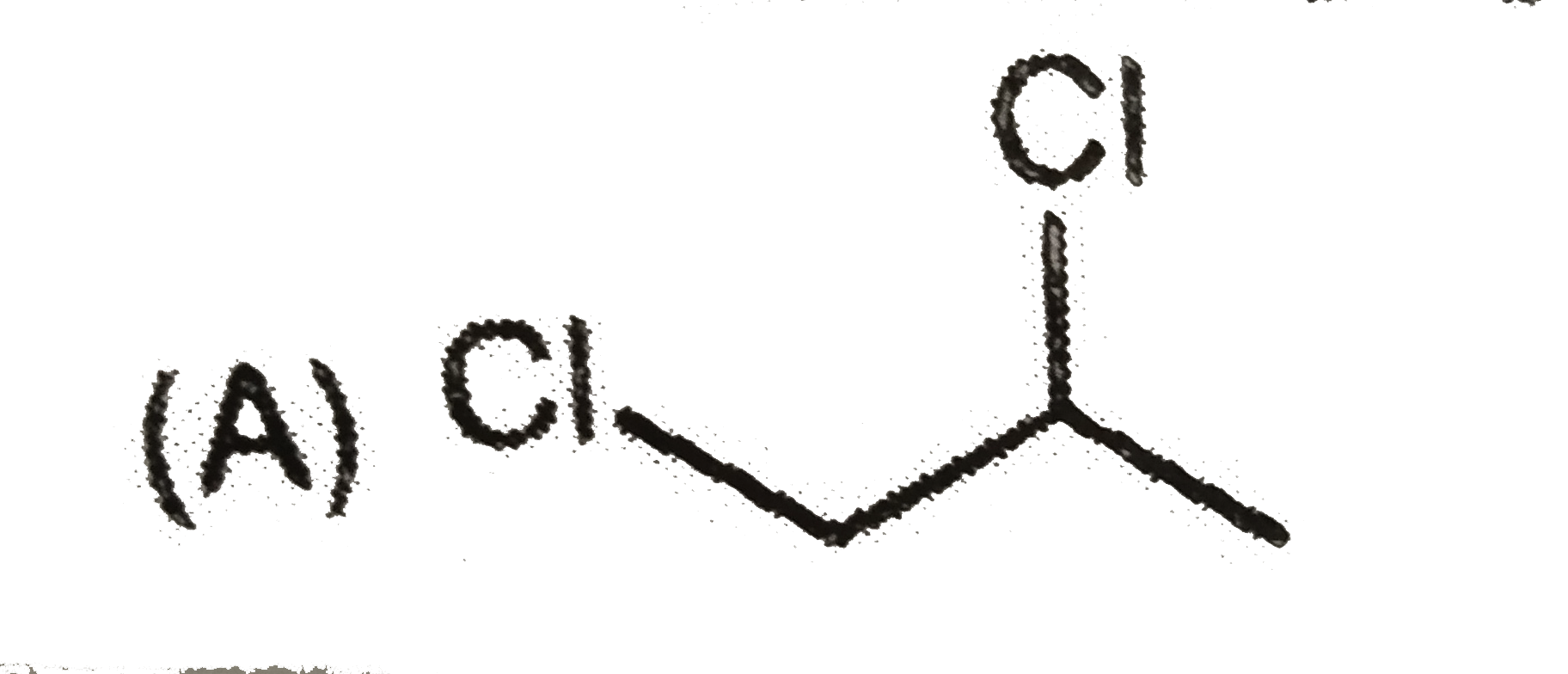
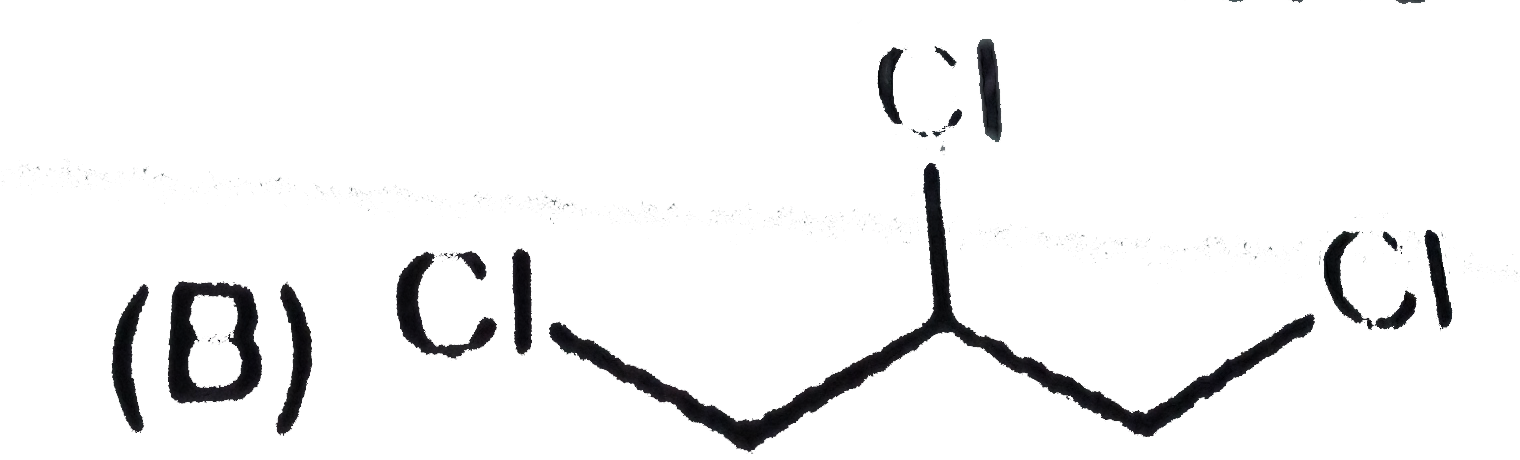
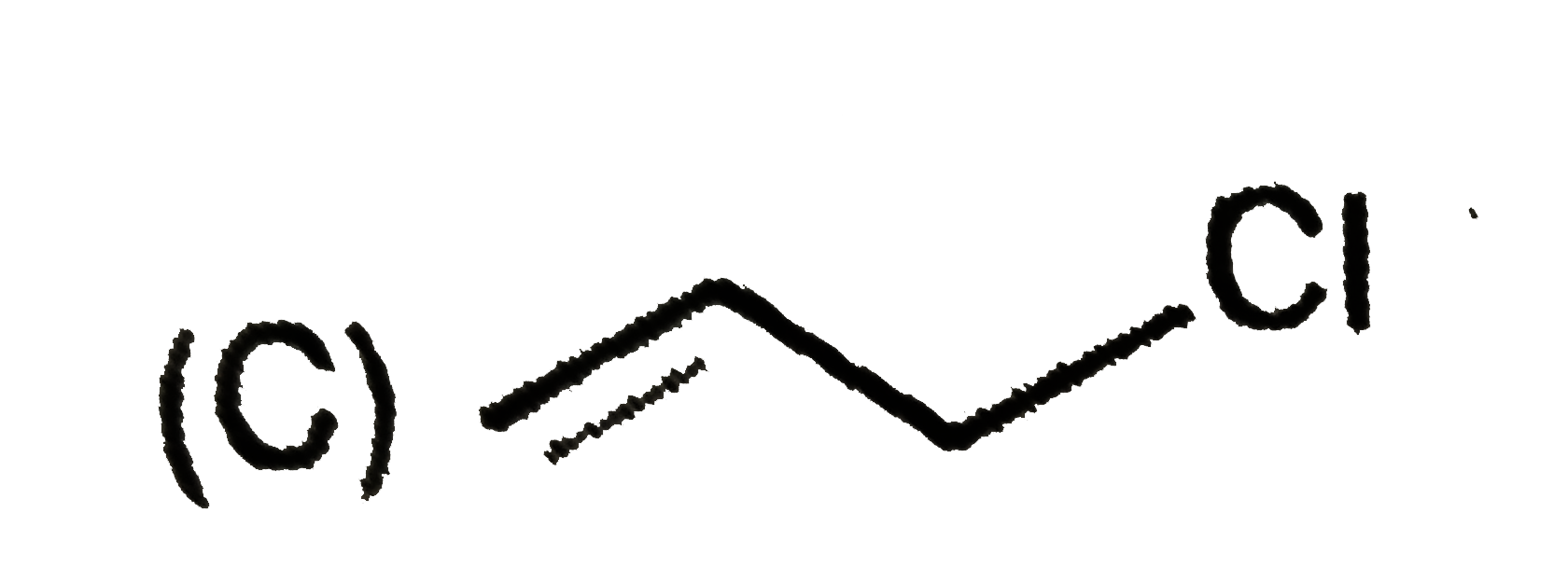
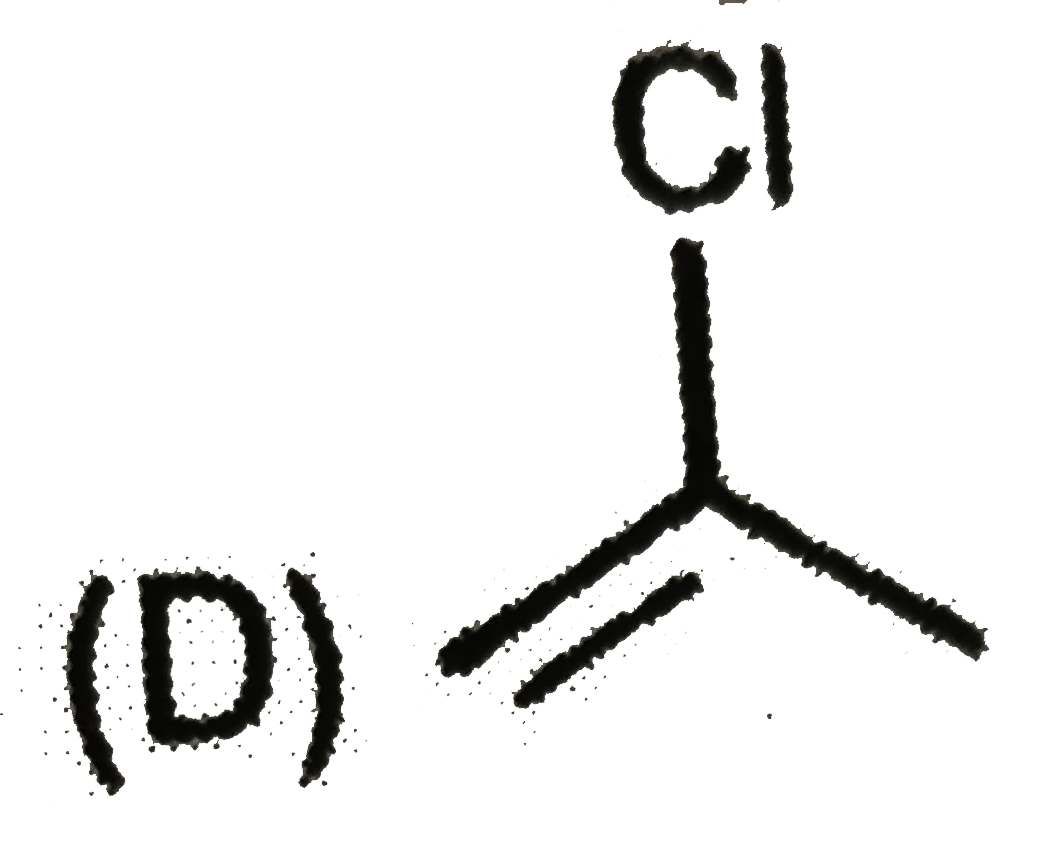
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise APSP Part - 3|22 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise APSP Part - 1|30 VideosMOLE CONCEPT
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS - II-APSP Part - 2
- Which compound amongst the following is nitrated with most difficulty ...
Text Solution
|
- The reaction of toluene with CI(2) in presence of FeCI(3) gives predom...
Text Solution
|
- In the reaction of chlorine with propene at 450^@C, the major product ...
Text Solution
|
- In the nitration of an aromatic compound using a mixture of concentrat...
Text Solution
|
- Select the major product obtained from the addition of HBr to 1-methyl...
Text Solution
|
- Reaction of benzene with isobutylchloride (CH3CH(CH3)CH2Cl) in the pre...
Text Solution
|
- The reagent system for preparing propan-1-ol from propene is :-
Text Solution
|
- In Friedel - Craft acylation the amount of AlCl3 that must be taken is
Text Solution
|
- For a Friedel - Craft reaction using AlCl3which compound can be used a...
Text Solution
|
- Predict the product of the reaction given below : CH3-CH2-CH=CH2und...
Text Solution
|
- Which can not be the major product formed upon addition of 1 mole o...
Text Solution
|
- Predict the product formed in the following reaction
Text Solution
|
- What is the major product that will be formed in the following reactio...
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- Identify the alkene which will not provide the following alcohol upon ...
Text Solution
|
- The compound X in the reaction.
Text Solution
|
- Cyclohexene reacts with limited amount of bromine in the presence of l...
Text Solution
|
- The major product of the following reaction is : (CH3)2C=CH-CH2-CH3o...
Text Solution
|
- The compound which does not react with bromine easily at room temperat...
Text Solution
|
- Major product of mononitration of the following compound is :
Text Solution
|