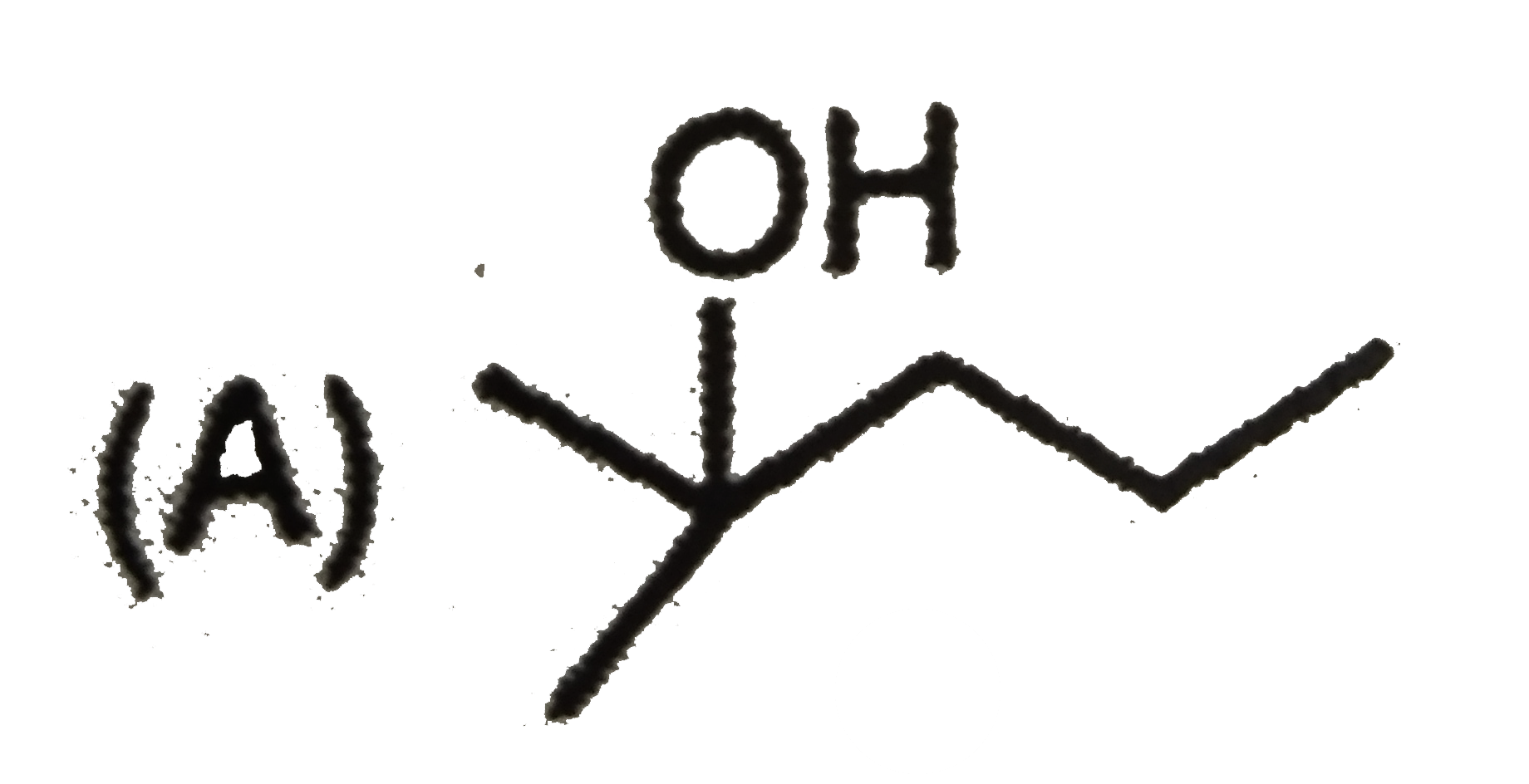
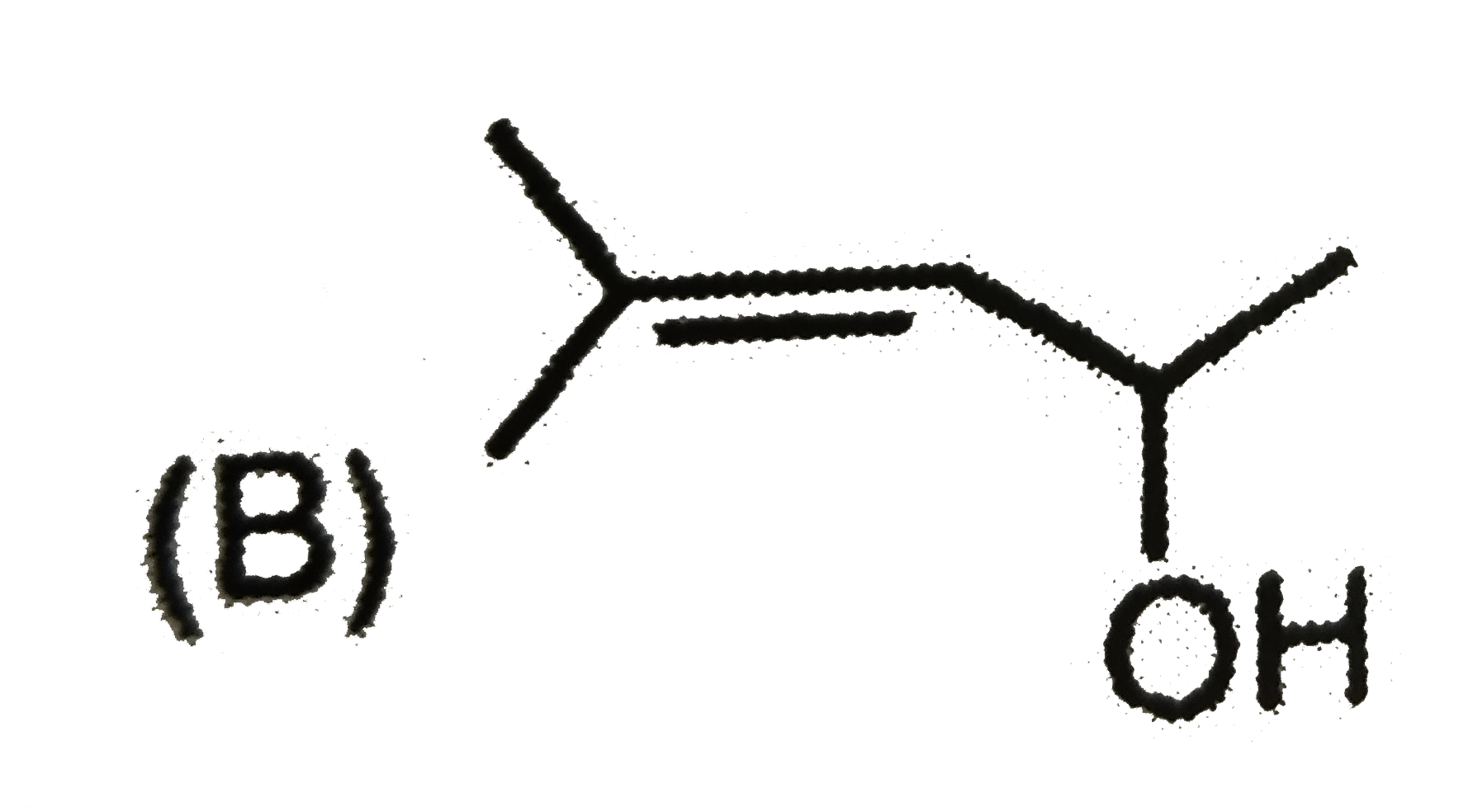
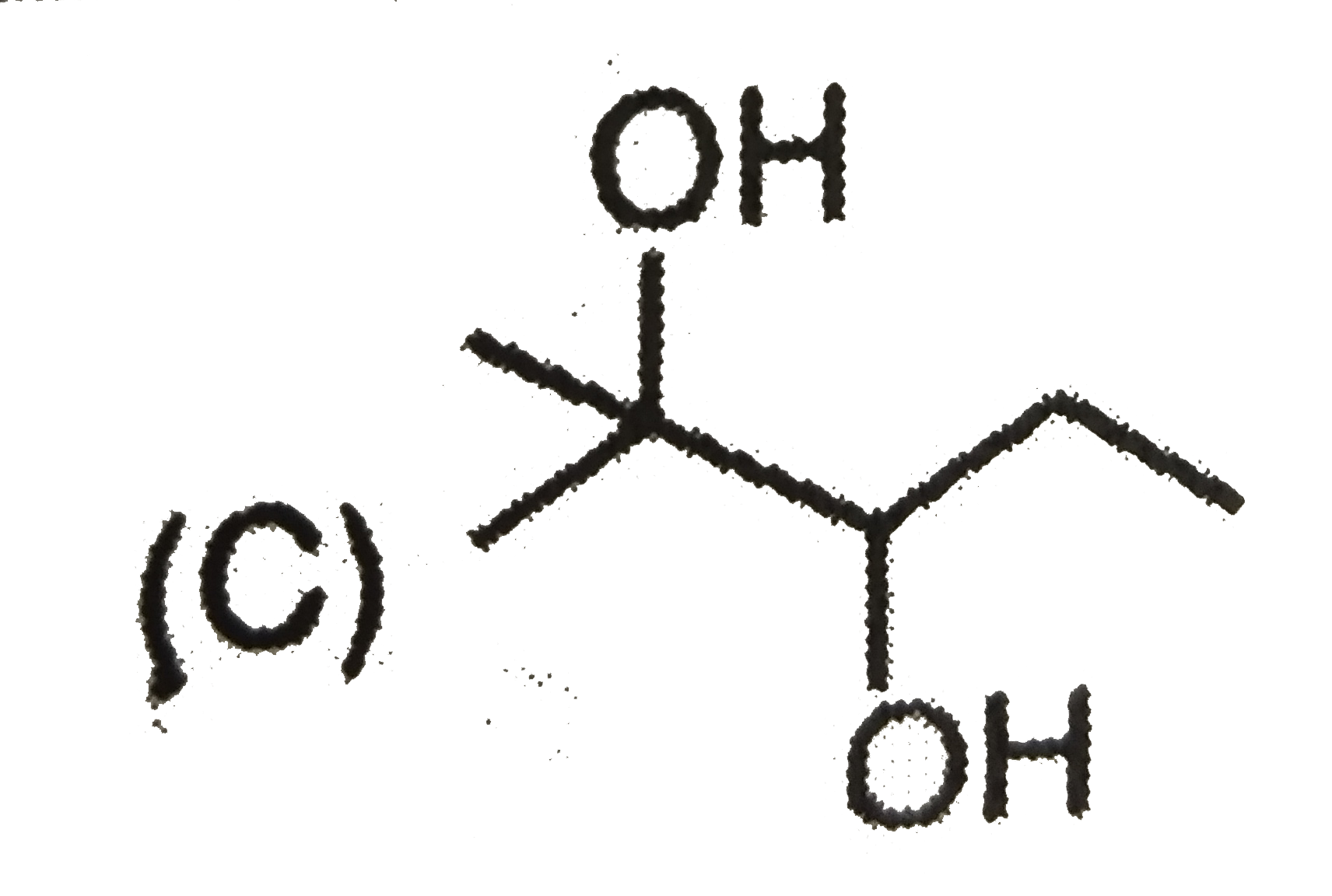
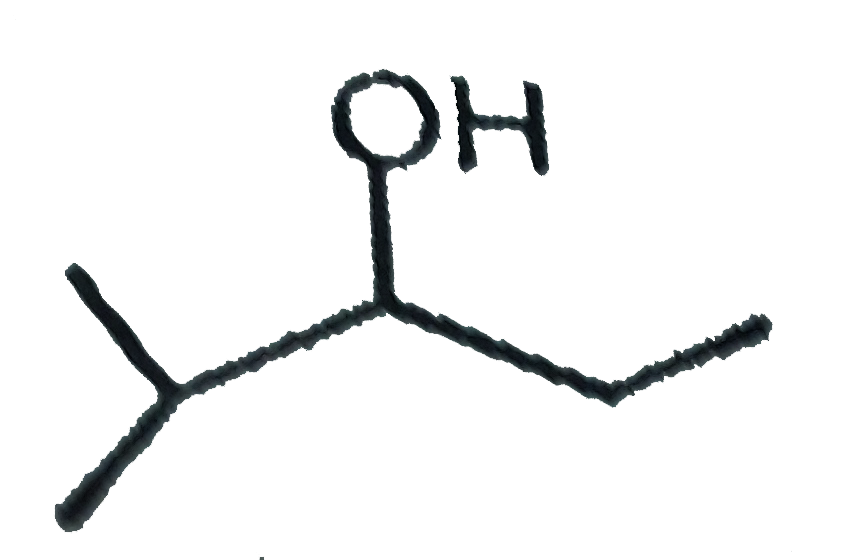
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise APSP Part - 3|22 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise APSP Part - 1|30 VideosMOLE CONCEPT
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-3|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS - II-APSP Part - 2
- The compound X in the reaction.
Text Solution
|
- Cyclohexene reacts with limited amount of bromine in the presence of l...
Text Solution
|
- The major product of the following reaction is : (CH3)2C=CH-CH2-CH3o...
Text Solution
|
- The compound which does not react with bromine easily at room temperat...
Text Solution
|
- Major product of mononitration of the following compound is :
Text Solution
|
- The product obtained from the following sequence of reactions is H(3...
Text Solution
|
- The product (P) of the following reaction is:
Text Solution
|
- Which isomer of xylene can give three different monochloroderivatives ...
Text Solution
|
- The 'product' in the above reaction is :
Text Solution
|
- (I) The rate of o-nitration of the above compounds, (I) toluene, (II...
Text Solution
|
- The correct name of the product obtained is
Text Solution
|
- Which of the following statements is correct ?
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- The product 'N' of the following reaction is
Text Solution
|
- The best sequence of reactions for the following conversion is
Text Solution
|
- 1,3-pentadiene and 1,4-pentadiene are compared with respect to their i...
Text Solution
|
- The hydrocarbon that cannot be prepared effectively by Wurtz reaction ...
Text Solution
|
- The reaction of 1-phenylpropane with limited amount of chlorine in the...
Text Solution
|
- 3-Methylpentane on monochlorination gives four possible products. The ...
Text Solution
|
- The best sequence of reactions for preparation of the following compou...
Text Solution
|