A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS - II-APSP Part - 3
- Complete the following reaction
Text Solution
|
- The reaction of toluene with CI(2) in presence of FeCI(3) gives X and ...
Text Solution
|
- The correct order of heat of combustion for following alkadienes is
Text Solution
|
- X,Y and Z reaction are :
Text Solution
|
- The major product of the given reaction is :
Text Solution
|
- In which reaction incorrect products have been reported.
Text Solution
|
- In the chlorination of Methane which of the following reaction involve...
Text Solution
|
- Which of the following reactions are completed through free radical in...
Text Solution
|
- Which of the following statement are correct for give reaction. CH3-...
Text Solution
|
- In which of the following reactions and products are correctly matched...
Text Solution
|
- Which statement is/are correct.
Text Solution
|
- Which of the following statements is/are incorrect ?
Text Solution
|
- How many of the following substituents can cause aromatic electrophili...
Text Solution
|
- How many alkene/s react faster than propane with dil. H(2)SO(4)?
Text Solution
|
- When addition of Br2 was carried out in presence of aq. NaCl on ethene...
Text Solution
|
- How many reactions will proceed through free radical addition mechanis...
Text Solution
|
- In the given reactions M is the number of major products obtained in I...
Text Solution
|
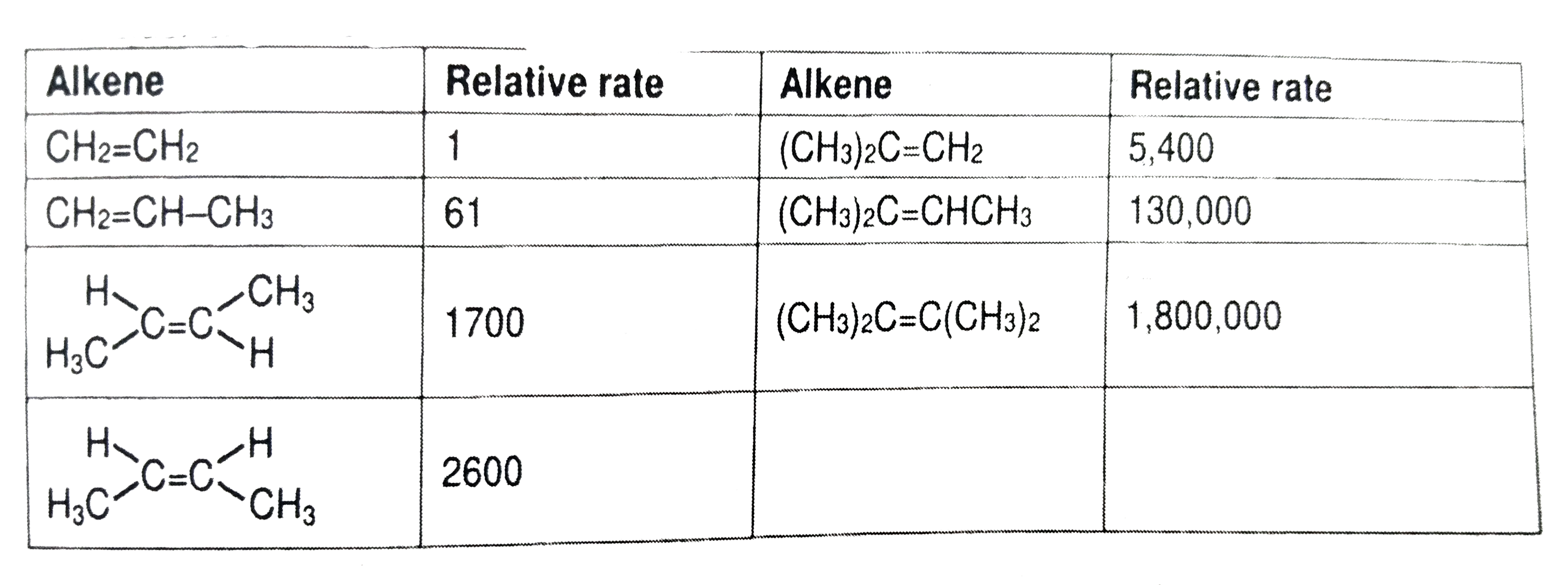
- Consider experimental data shown in the table : Rate of electroph...
Text Solution
|
- Consider experimental data shown in the table : Which of the foll...
Text Solution
|
- Match List-I (Compounds) with List-II (% meta electrophilic substituti...
Text Solution
|