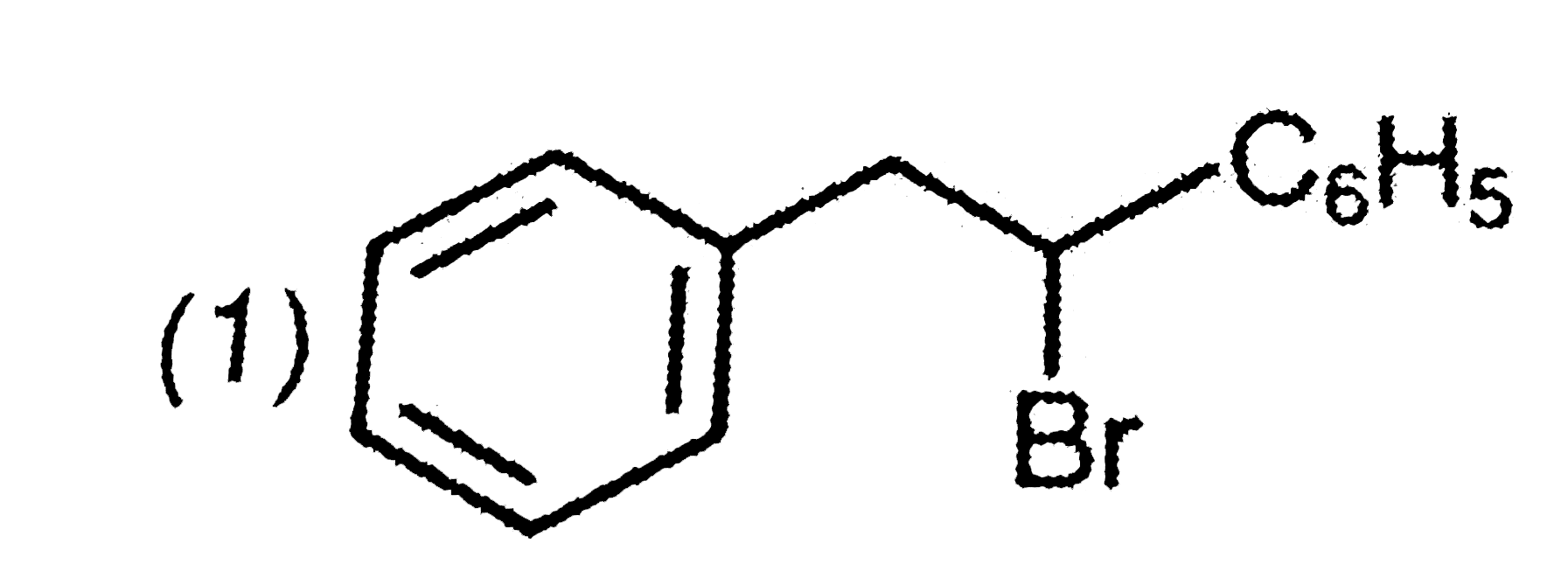
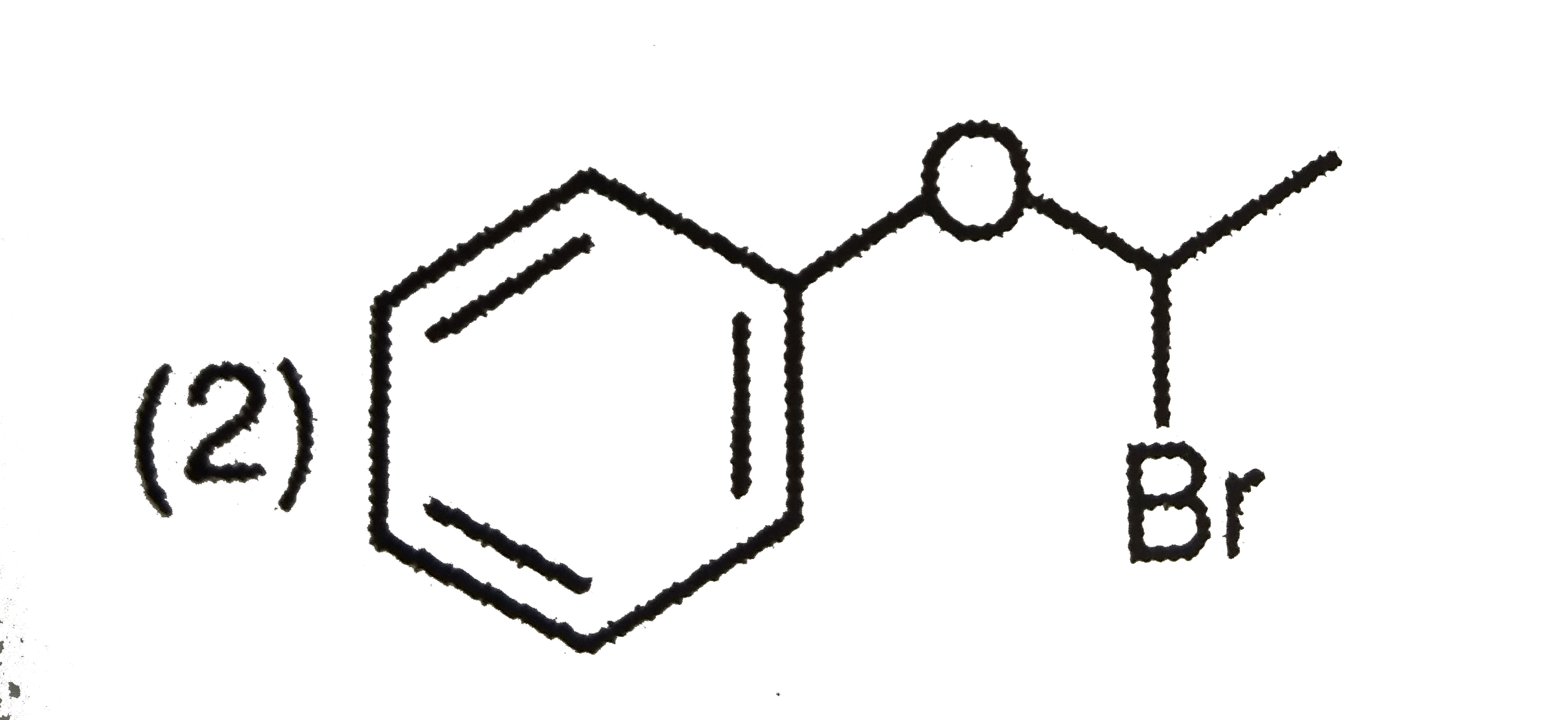
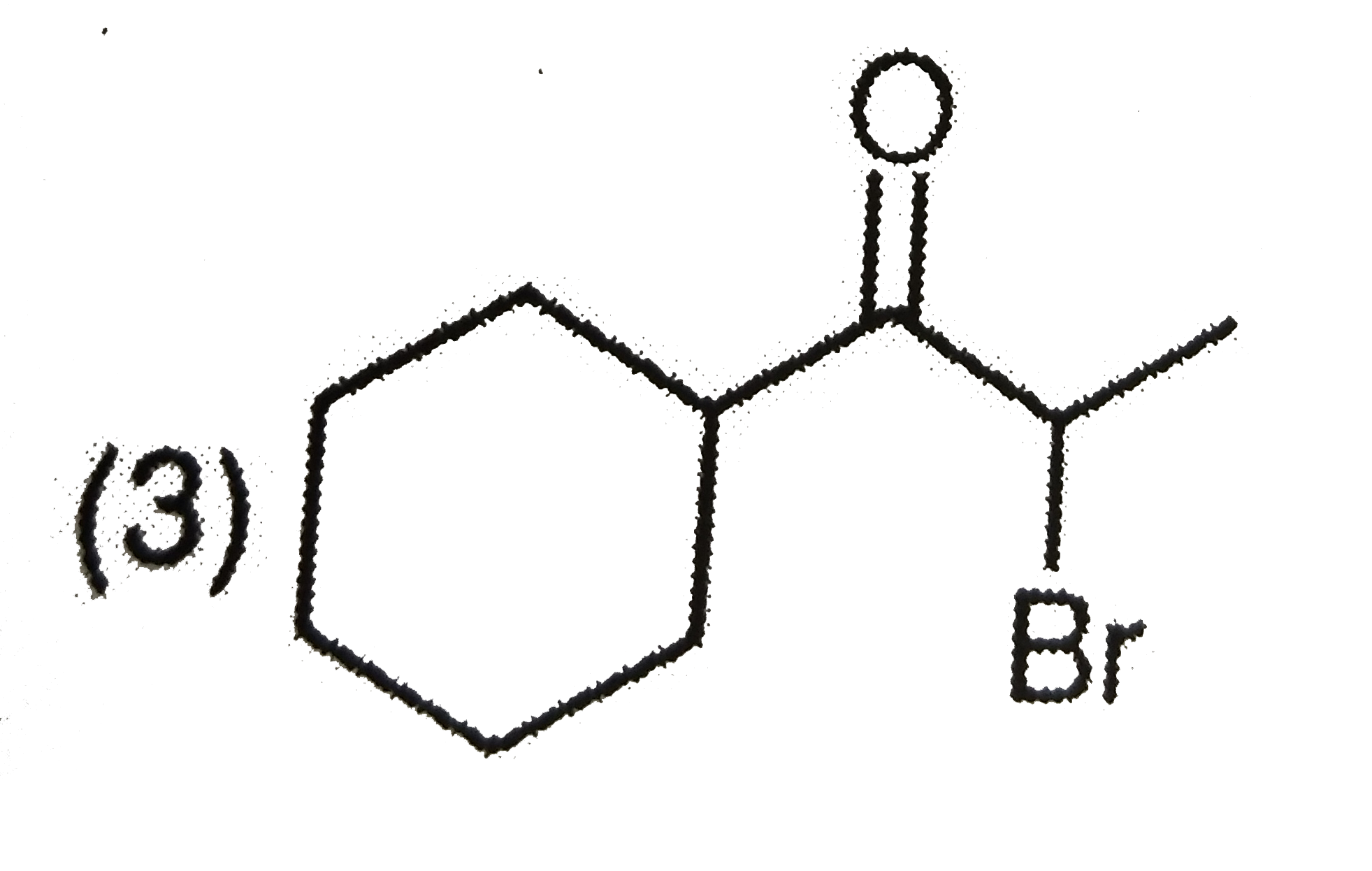
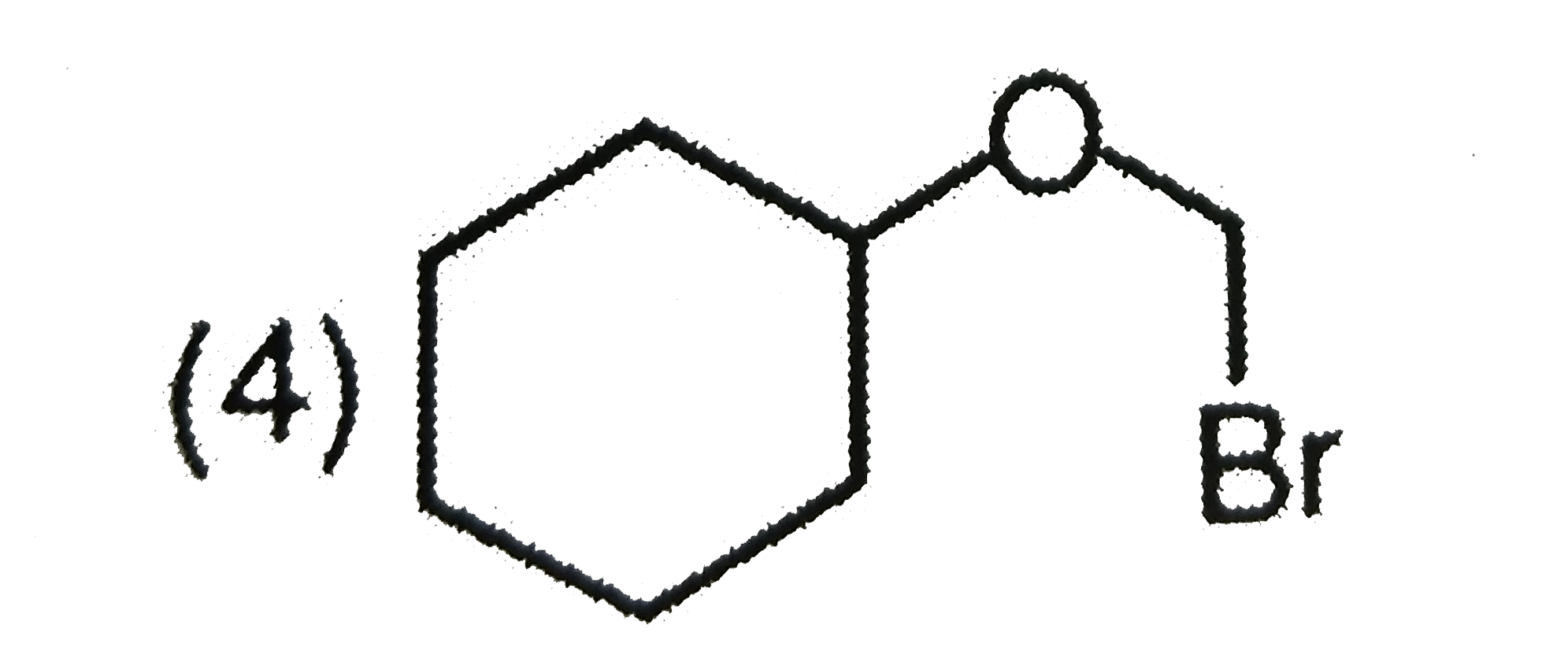
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise Exercise-3 Part-2 JEE (MAIN ) ONLINE PROBLEMS|10 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-1|30 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise Exercise-3 Part-1|18 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise APSP Part - 3|22 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS-IV-Exercise-3 Part-2 JEE (MAIN ) OFFLINE PROBLEMS
- Maximum dehydration takes place that of-
Text Solution
|
- During dehydration of alcohols to alkenes by heating with conc. H(2) S...
Text Solution
|
- Elimination of HBrfrom 2 bromobutane results in the formation of .
Text Solution
|
- Reaction of trans-2-phenyl-1-bromocyclopentane on reaction with alcoho...
Text Solution
|
- The alkene formed as a major product in the above elimination reaction...
Text Solution
|
- In the reaction CH(3)COOH overset(LiAIH(4))rarr A overset(PCl(g))rar...
Text Solution
|
- 2-Chloro-2-methylpentane on reaction with sodium methoxide in methanol...
Text Solution
|
- The major product obtained in the following reaction is :
Text Solution
|
- Which of the following , upon treatment with tert-BuONa followed by ad...
Text Solution
|
- The major product of the following reaction is :
Text Solution
|