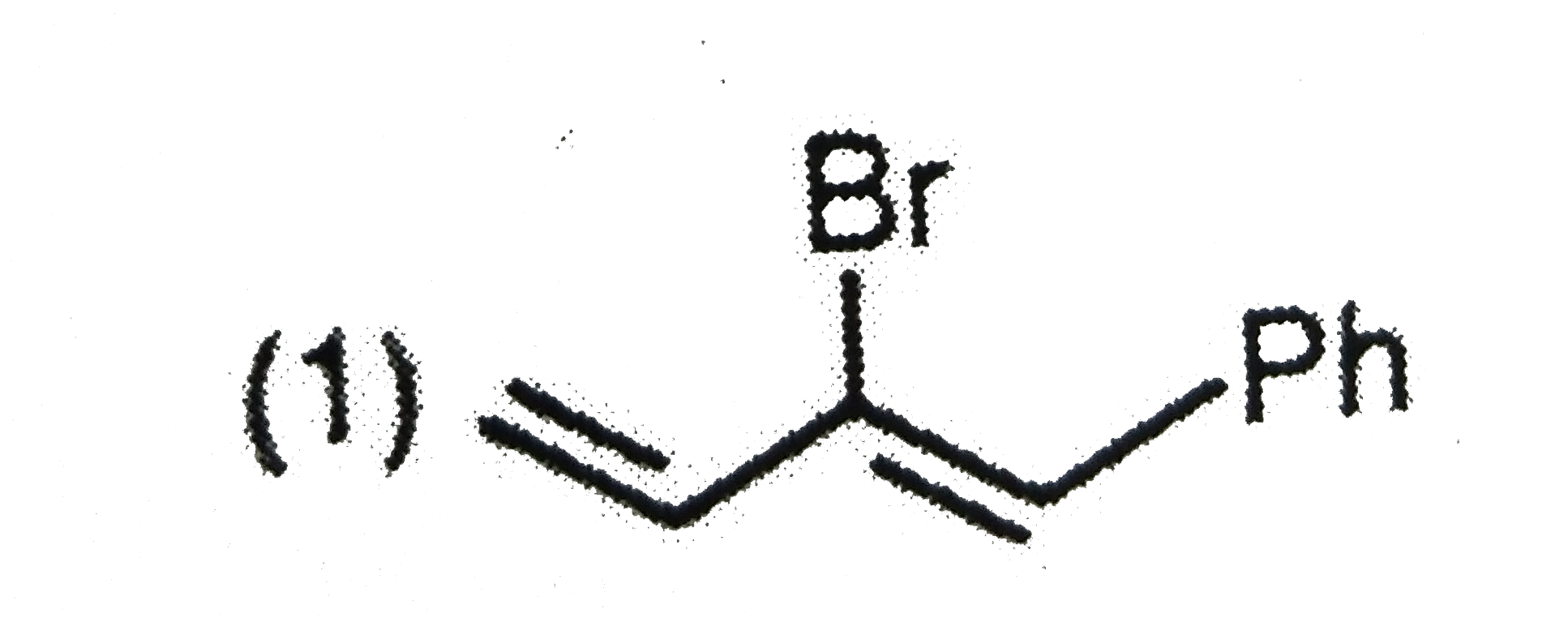
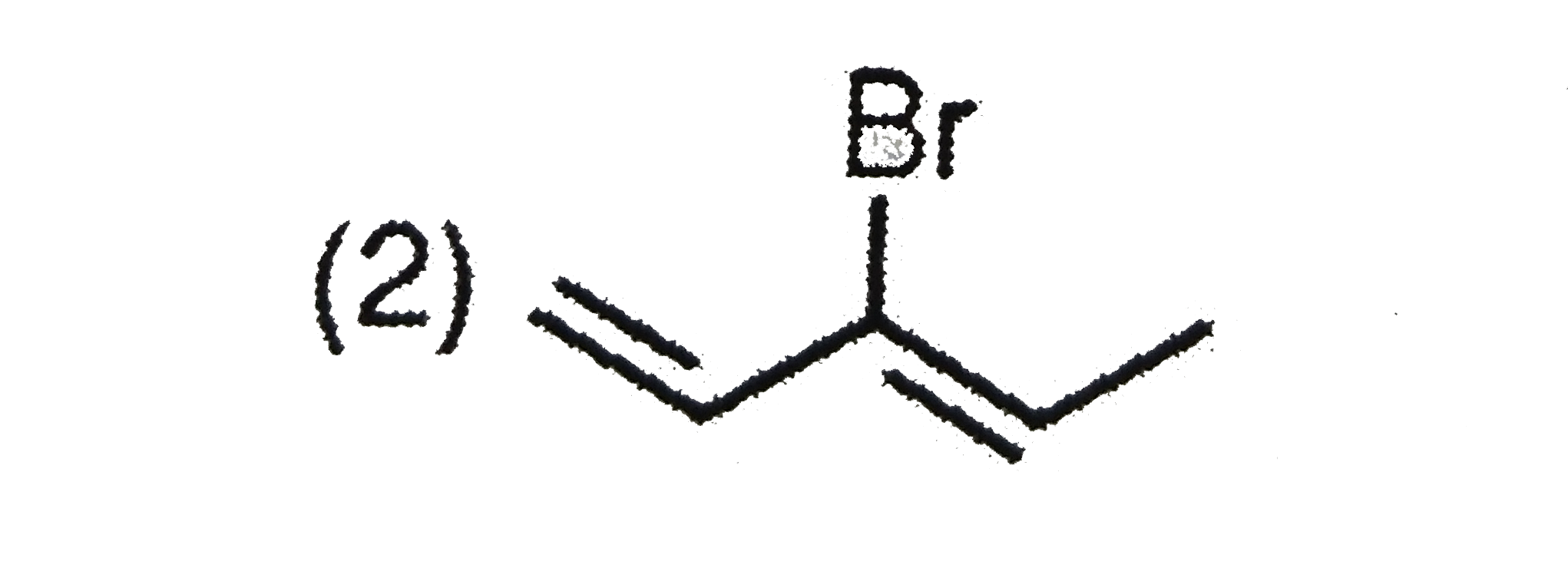
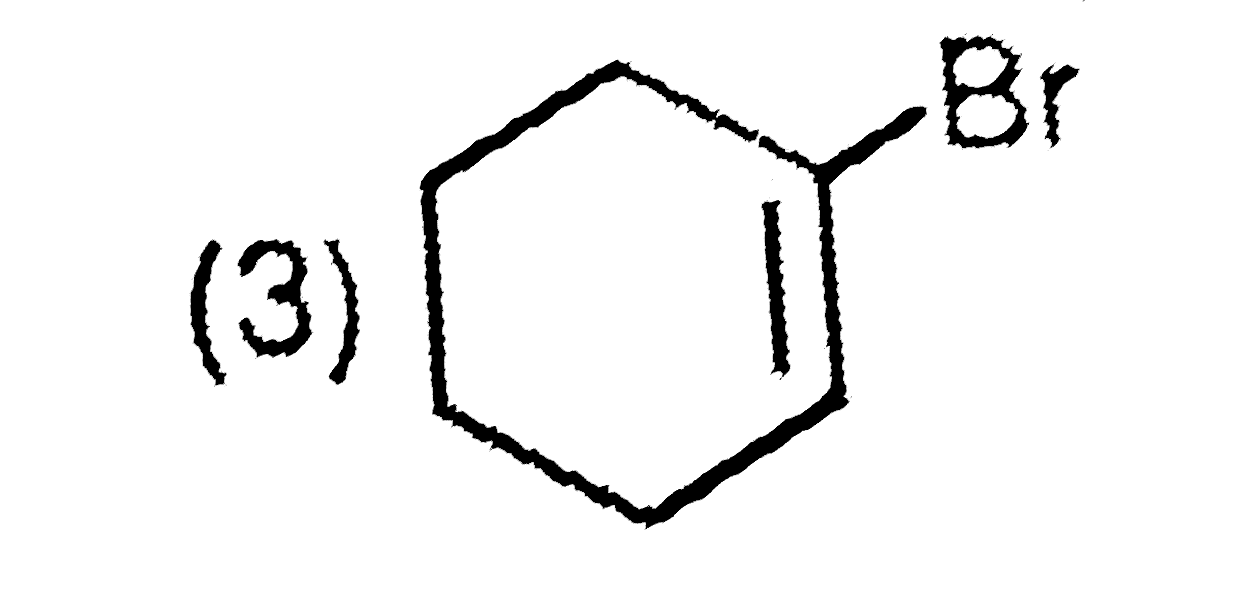
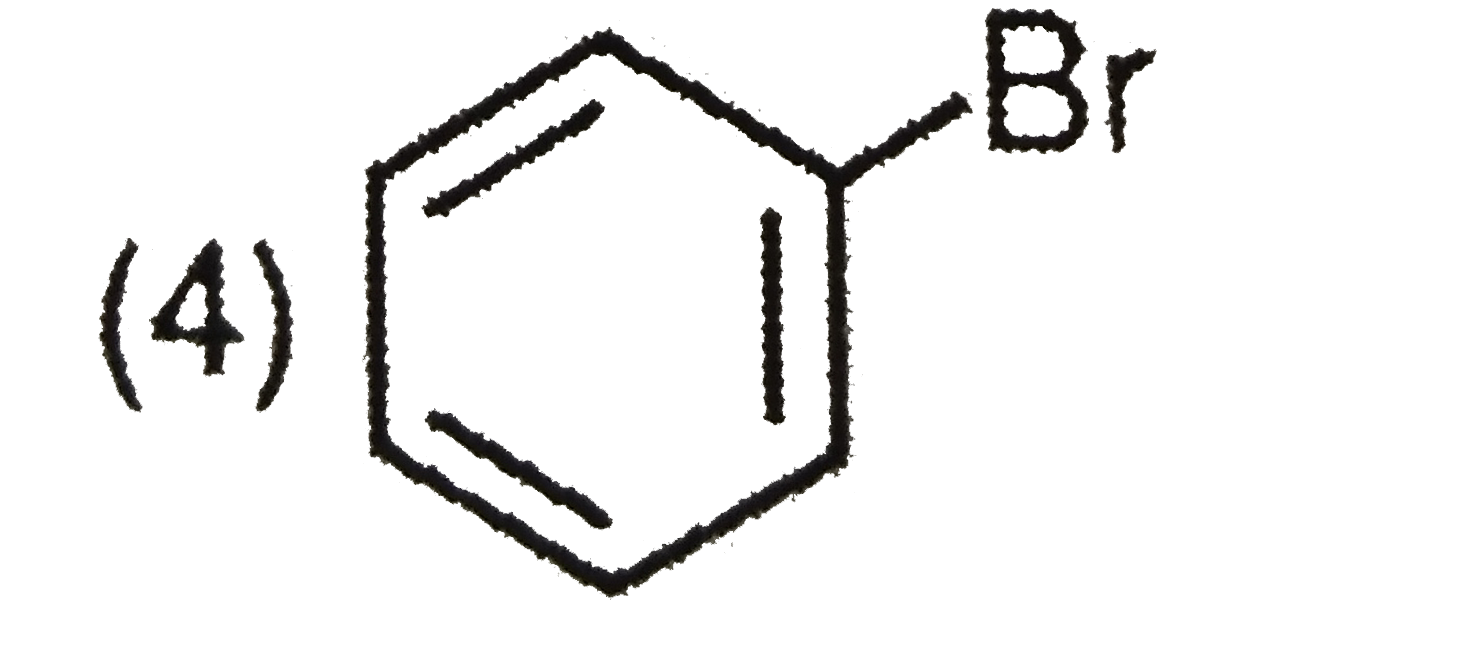
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-1|30 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-2|17 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise Exercise-3 Part-2 JEE (MAIN ) OFFLINE PROBLEMS|10 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise APSP Part - 3|22 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS-IV-Exercise-3 Part-2 JEE (MAIN ) ONLINE PROBLEMS
- The major product of the reaction is
Text Solution
|
- Which one of the following reagents is not suitable for the eliminatio...
Text Solution
|
- The major product of the following reaction is : CH(3)underset(overs...
Text Solution
|
- The major product of the following reaction is : C6H5CH2-undersetund...
Text Solution
|
- Which of the following will most readily give the dehydrohalogenation ...
Text Solution
|
- The major product formed in the following reactions is :
Text Solution
|
- Which of the following compounds will most readily be dehydrated to gi...
Text Solution
|
- The major product of the following reaction is :
Text Solution
|
- The major product of the following reaction is : CH(3)CH(2)underset(...
Text Solution
|
- The major product of the following reaction is :
Text Solution
|