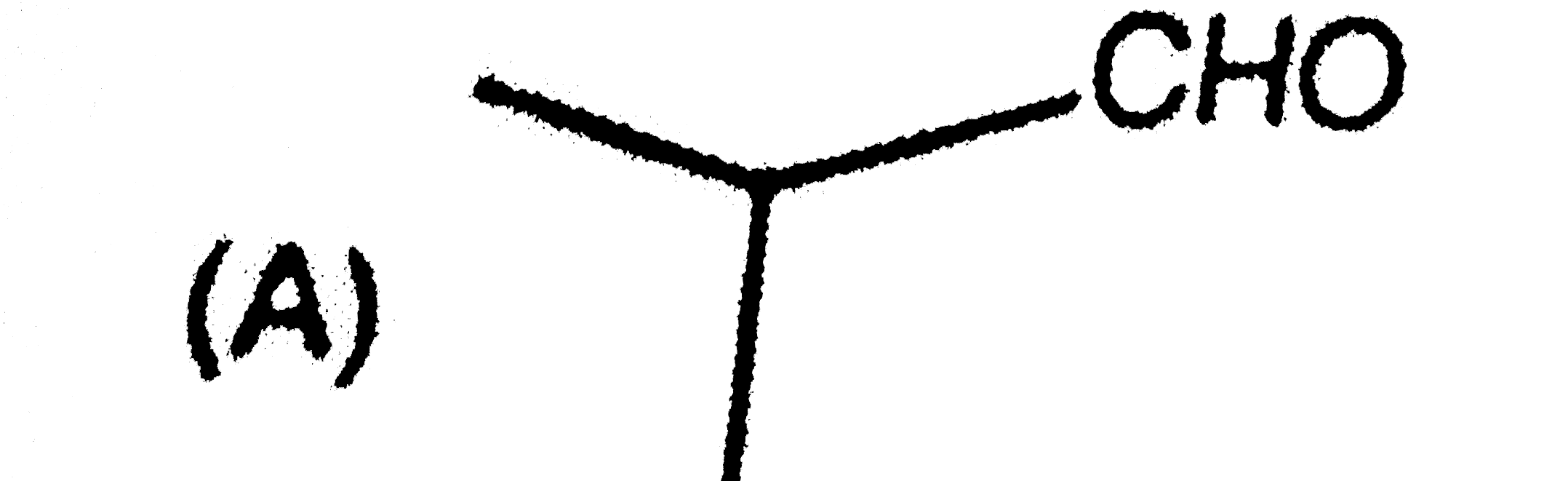
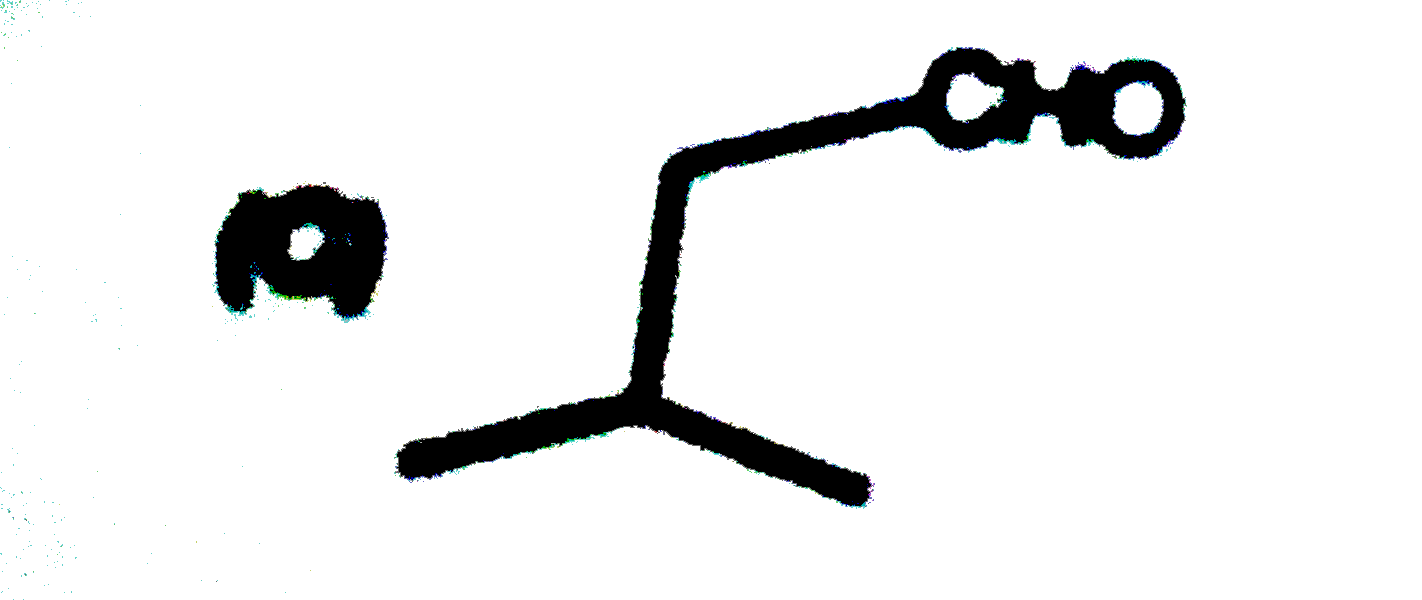
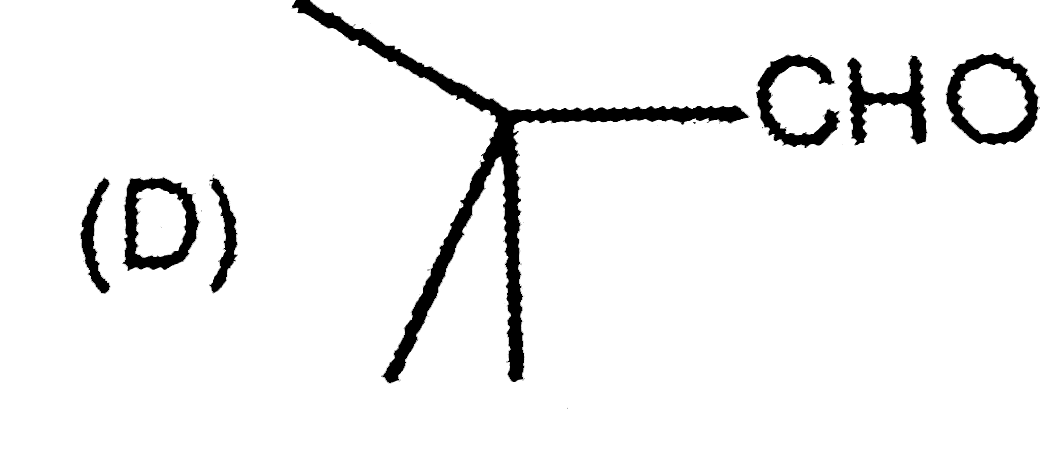
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-3|22 VideosORGANIC REACTION MECHANISMS-IV
RESONANCE ENGLISH|Exercise APSP PART-1|30 VideosORGANIC REACTION MECHANISMS - II
RESONANCE ENGLISH|Exercise APSP Part - 3|22 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS-IV-APSP PART-2
- An alkyl bromide produces a single alkene when it reacts with sodium e...
Text Solution
|
- Which of the following most readily undergoes E(2) elimination with a ...
Text Solution
|
- An alkyl chloride produces a single alkene on reaction with sodium eth...
Text Solution
|
- On heating glycerol with conc. H(2)SO(4) ,a compound is obtained whic...
Text Solution
|
- n-propyl bromide on treatment with ethanolic potassium hydroxide produ...
Text Solution
|
- The peroxide effect occurs by :
Text Solution
|
- Acid catalysed dehydration of 2-pentanol would give
Text Solution
|
- The major product formed in the following reactions is :
Text Solution
|
- Arrange the following compounds in order of decreasing reactivity in ...
Text Solution
|
- Compound X on treatment with HI give Y, Y on treatment with ethanolic ...
Text Solution
|
- The major product of the following reactions is :
Text Solution
|
- The carbanion expels a leaving group LG to yield an alkene as shown ab...
Text Solution
|
- The compound that is most reactive with alcoholic KOH is
Text Solution
|
- Four processes are indicated below : The processes that do not pr...
Text Solution
|
- n-Butylcyclohexane is formed through the following sequence of reactio...
Text Solution
|
- An alkyl halide (X) on reaction with ethanolic sodium hydroxide forms ...
Text Solution
|
- Spodoptol, a sex attractant, produced by a female fall armyworm moth, ...
Text Solution
|