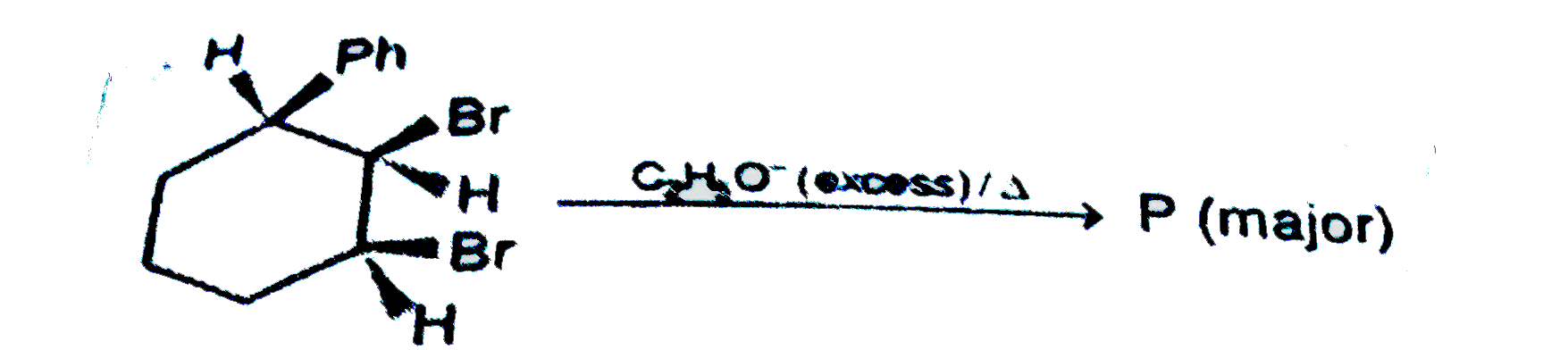
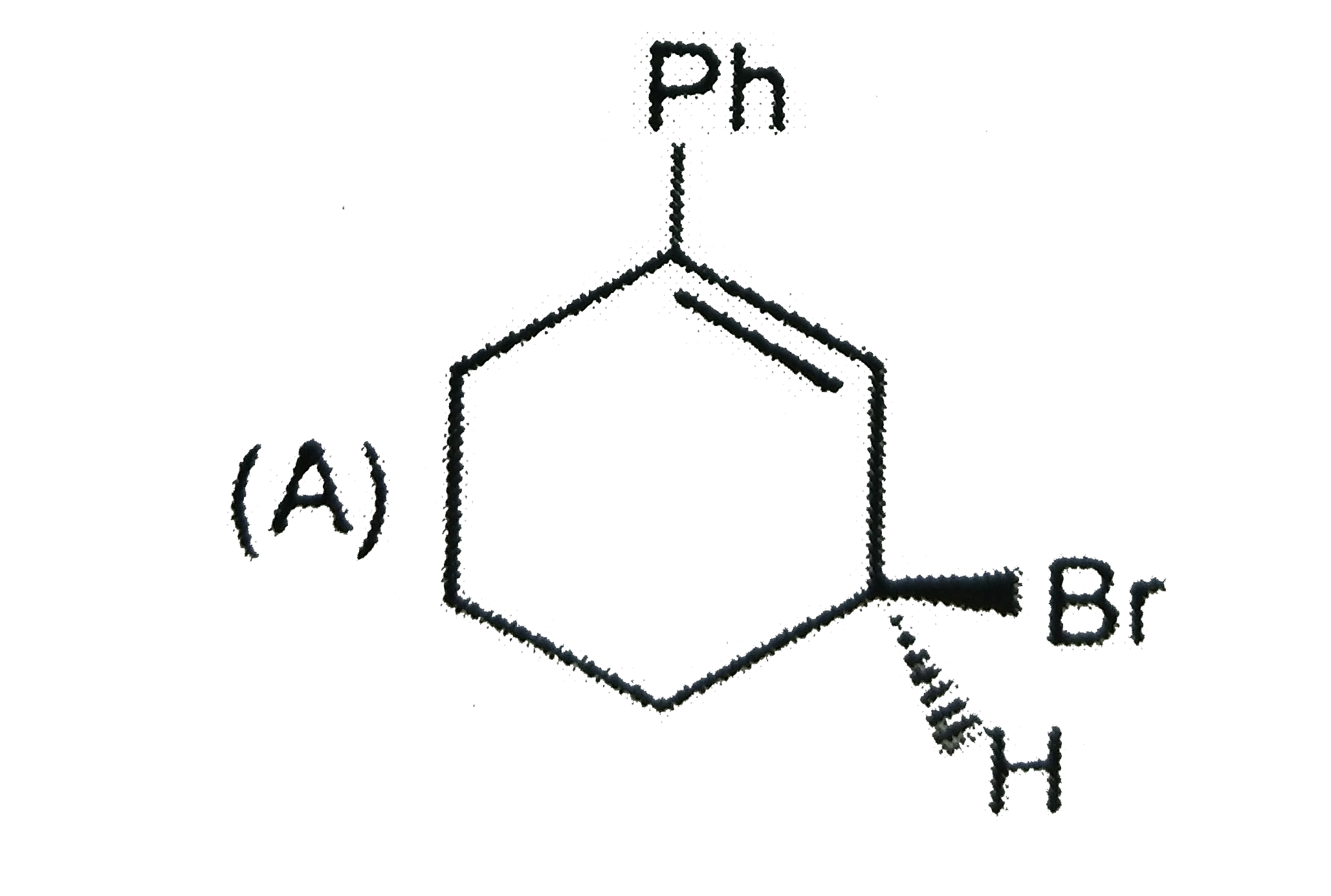
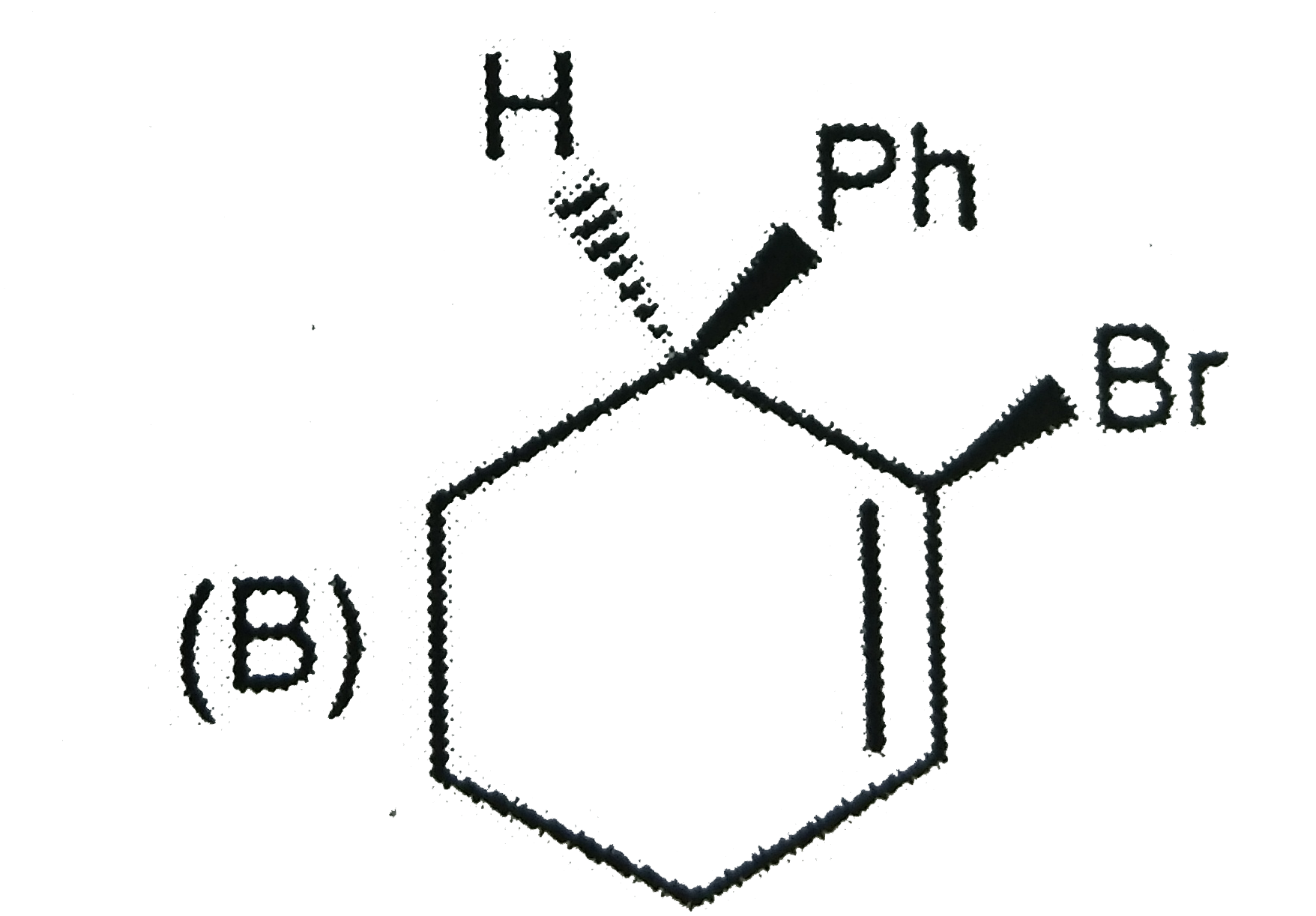
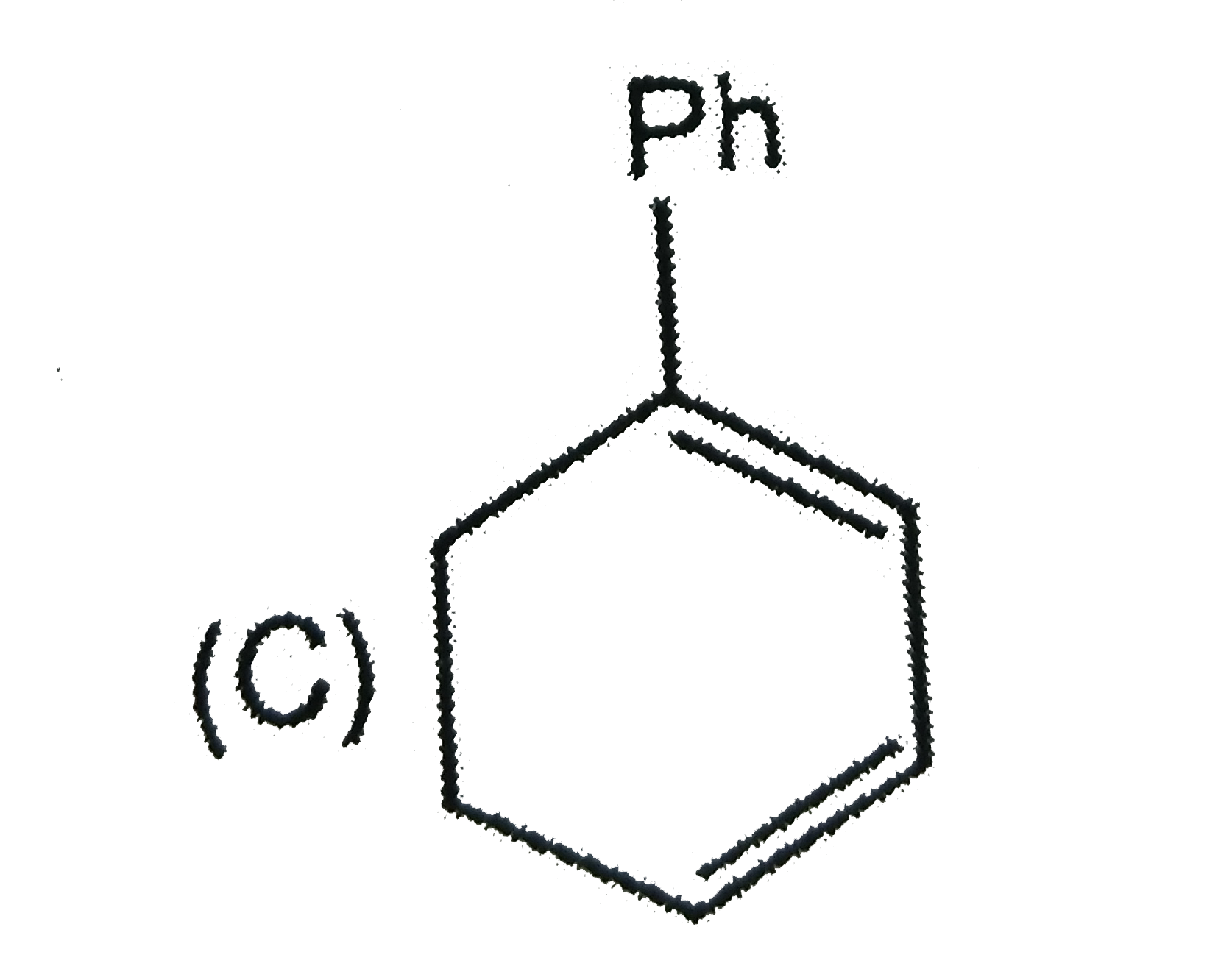
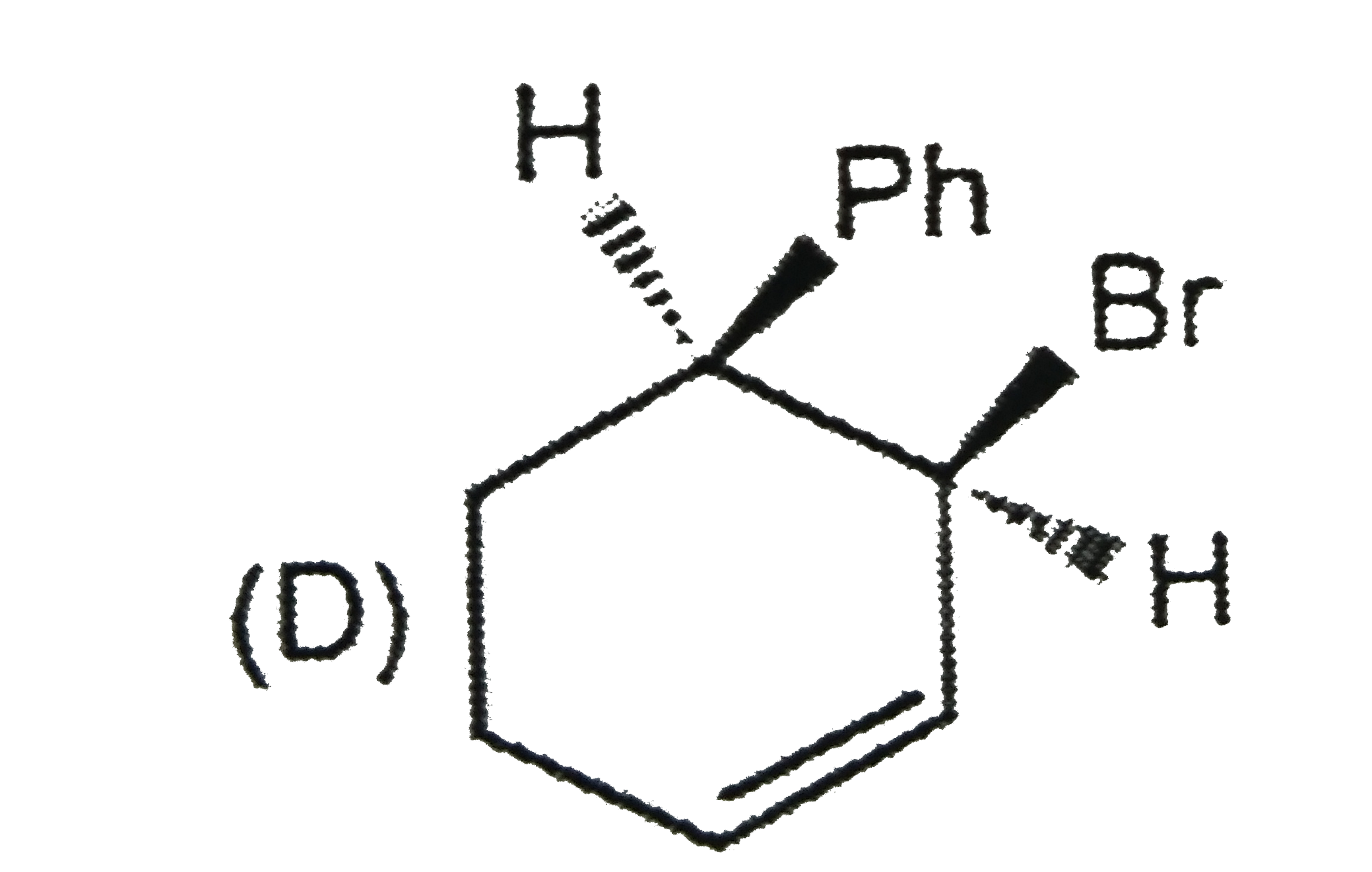
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-ORGANIC REACTION MECHANISMS-IV-APSP PART-3
- The compound 'X' is
Text Solution
|
- When the all-cis isomer of C6H6Cl6 (1,2,3,4,5,6 -Hexachlorohexane) is ...
Text Solution
|
- CH2Ounderset(H3O^(o+))overset(CHD2MgI)toX underset(Delta)overset(Conc....
Text Solution
|
- Major product of the given reaction is :
Text Solution
|
- The product is -
Text Solution
|
- Which one of the following hyxachlorocyclohexane is least reactive and...
Text Solution
|
- In which of the following reactions the correct major products are men...
Text Solution
|
- When ethyl bromide is added to potassium t-butoxide, the product is et...
Text Solution
|
- Which of the following statement are true ?
Text Solution
|
- Which of the following reaction has the correct major product .
Text Solution
|
- Which of the following statements is/are correct for alkyl halide ?
Text Solution
|
- The correct statements about the following reaction are [Hint: Al...
Text Solution
|
- The order of following reaction is :
Text Solution
|
- Total number of alkenes obtained by dehydration of 3,4-diethylhexan-2-...
Text Solution
|
- Report your answer as XY
Text Solution
|
- The number of products (stereoisomers) formed in the following reactio...
Text Solution
|
- The elimination reactions mainly involve three mechanism E1,E2 and E1c...
Text Solution
|
- The elimination reactions mainly involve three mechanism E1,E2 and E1c...
Text Solution
|
- The elimination reactions mainly involve three mechanism E1,E2 and E1c...
Text Solution
|
- Match List I (Reaction) with List II (Type of reaction ) and select th...
Text Solution
|