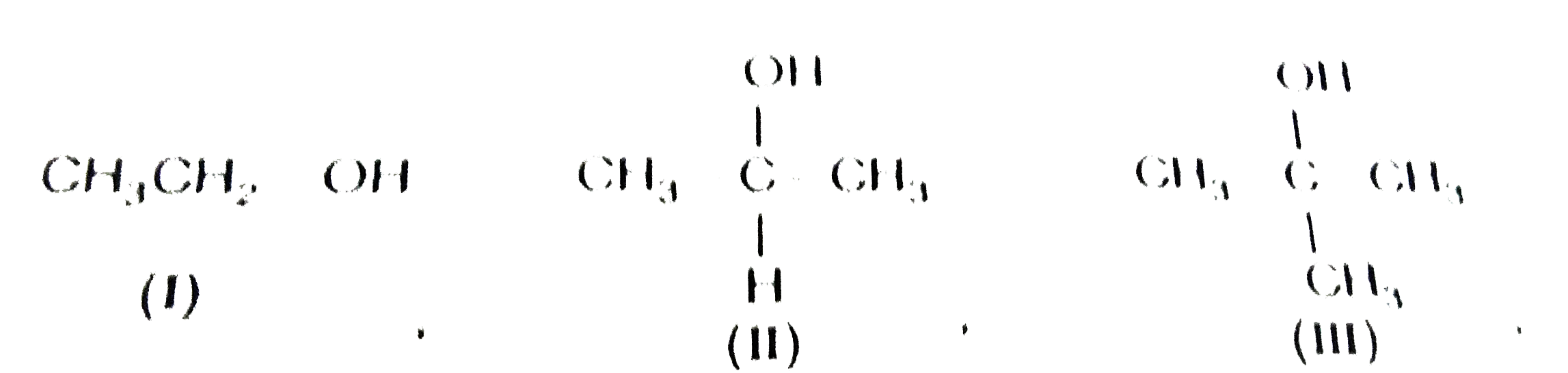A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
REDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Part-II: Single and Double Value Integer Type|5 VideosREDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Part-III: One or more than one options correct type|4 VideosREDUCTION, OXIDATION & HYDROLYSIS REACTIONS
RESONANCE ENGLISH|Exercise Part-III Match the column|2 VideosPERIODIC TABLE & PERIODICITY
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(BASIC CONCEPTS)|27 VideosS BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|4 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-REDUCTION, OXIDATION & HYDROLYSIS REACTIONS-Exercise-2 (Part-I: Only One option correct Type)
- {:(" "CHO),(" |"),(" ...
Text Solution
|
- In the above reaction the using reagents X and Y are :
Text Solution
|
- IUPAC names of the following compounds are
Text Solution
|
- Consider reduction of 2-butanone. A , B and C are respectively
Text Solution
|
- Identify P and (Q) respectively in the given reaction :
Text Solution
|
- Which of the following sets of compounds cannot turn clear orange solu...
Text Solution
|
- Product C is :
Text Solution
|
- The product which is not formed in the following reactions : {:(" ...
Text Solution
|
- Reagent P in the given reaction is :
Text Solution
|
- P and Q are respectively.
Text Solution
|