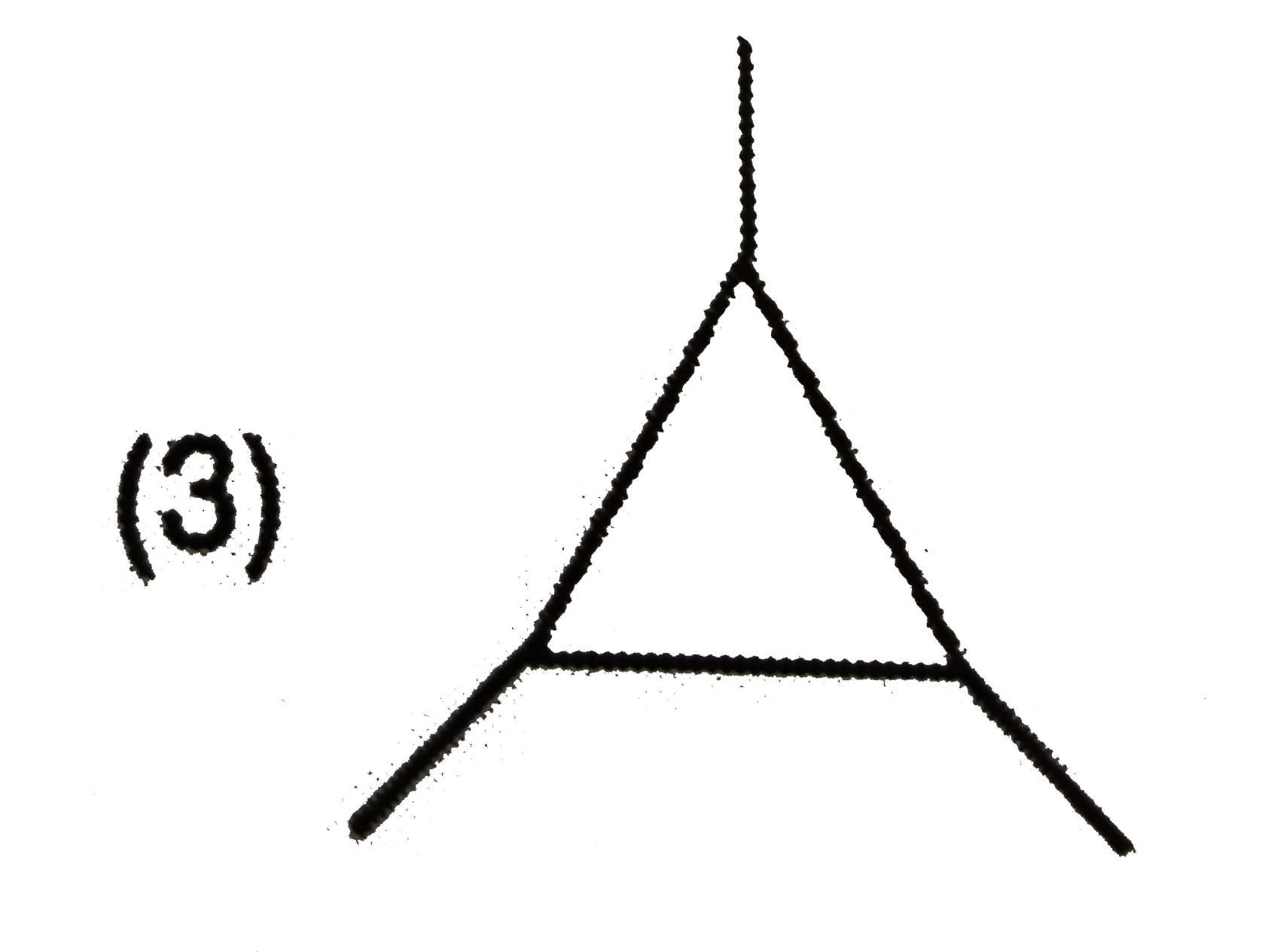
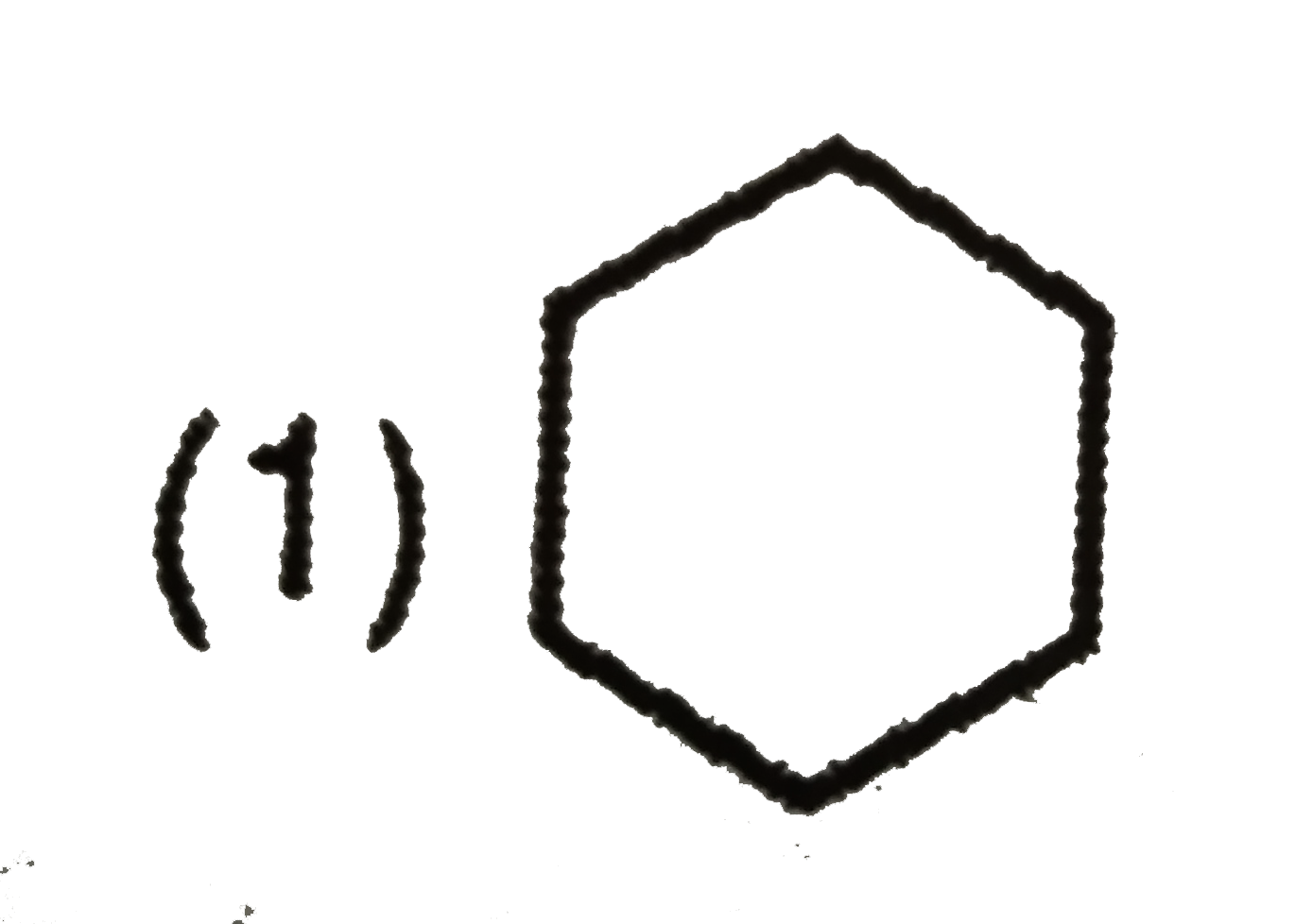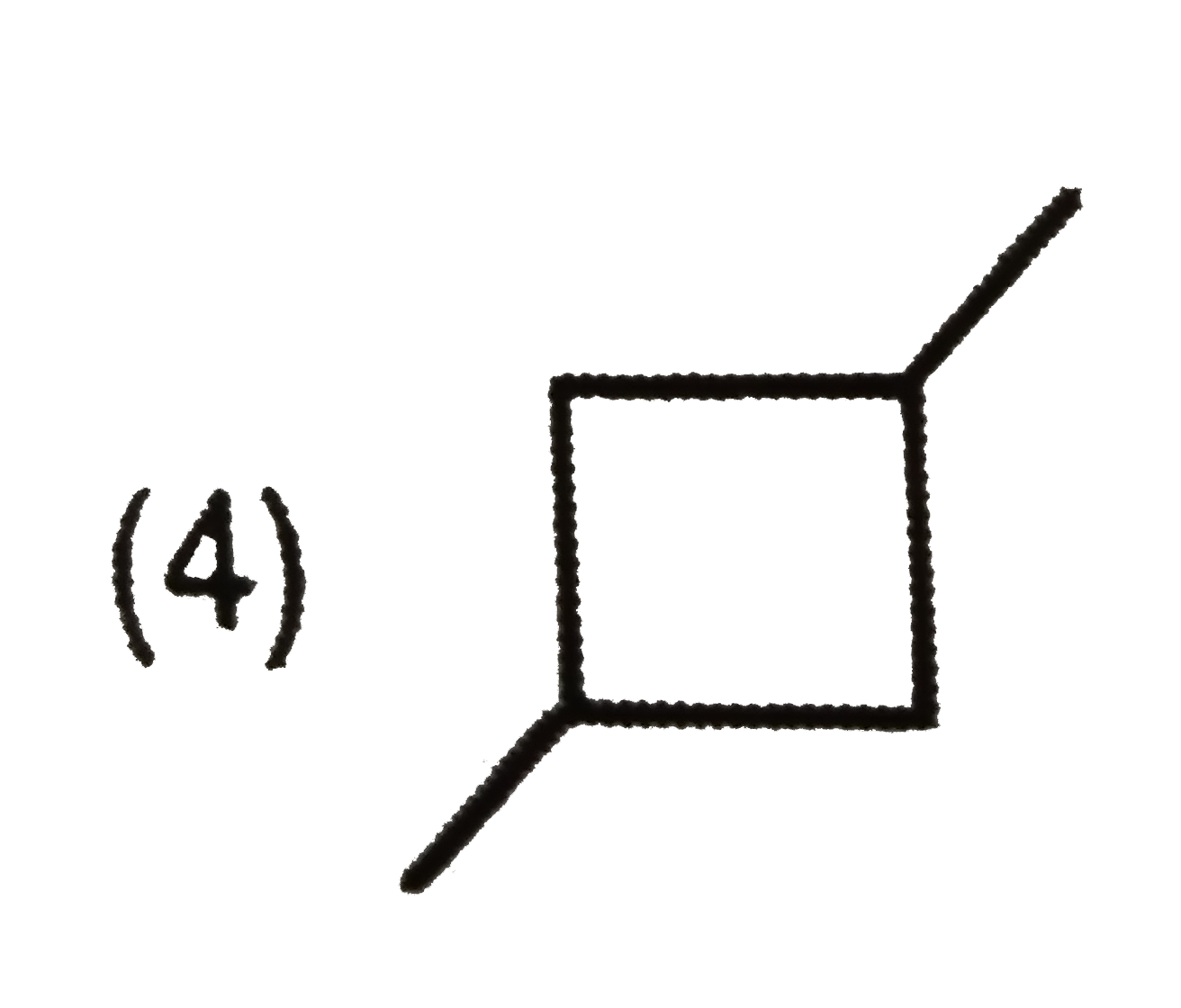A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE ENGLISH|Exercise Part-III|27 VideosSTRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE ENGLISH|Exercise Additional theory|4 VideosSTRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE ENGLISH|Exercise Jee main|6 VideosSOLID STATE
RESONANCE ENGLISH|Exercise Part- IV|21 VideosTHERMODYNAMIC & THERMOCHEMISTRY
RESONANCE ENGLISH|Exercise INORGANIC CHEMISTRY(P-Block Elements)|26 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-STRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY-APSP
- Which of the following is not a condensation polymer?
Text Solution
|
- How many isomeric structural alkene on catalytic hydrogenation gives 3...
Text Solution
|
- Compound A(C6H(12)) does not absorb H(2) in presence of Ni. It forms t...
Text Solution
|
- Which alkyne will give 3-Ethyl heptane on catalytic hydrogenation.
Text Solution
|
- Compound 'A' gives and precipitate with Cu(2)Cl(2)//NH(4)OH solution a...
Text Solution
|
- An organic compound does not react appreciably with Lucas reagent but ...
Text Solution
|
- Which of the following compounds will give a positive iodoform test ?
Text Solution
|
- The following two compounds I and II can be distinguished by using rea...
Text Solution
|
- which of the compound give iodoform when react with IO^(-) (hypoiodit...
Text Solution
|
- How many structural isomeric ketones having molecular formula (C(5)H(1...
Text Solution
|
- Which of the structural isomeric ketones having molecular formula (C(5...
Text Solution
|
- can be distinguish by
Text Solution
|
- (x) C(7)H(12)underset(Me(2)S)overset(O(3))rarrP+Q Compound P respond...
Text Solution
|
- Total number of monochloro structure products
Text Solution
|
- Yellow precipitate obtained during the test of halogen by lassaigne's...
Text Solution
|
- A research scholar get a mixture of three product during an experiment...
Text Solution
|
- How many alcohols give immediate turbidity with Lucas reagent having m...
Text Solution
|
- Which of the following compound can give test with Tollen's reagent an...
Text Solution
|
- Which is incorrect match with respect to the reagent used for lab test...
Text Solution
|
- How many hydrocarbons having molecular mass 68 can give white precipit...
Text Solution
|