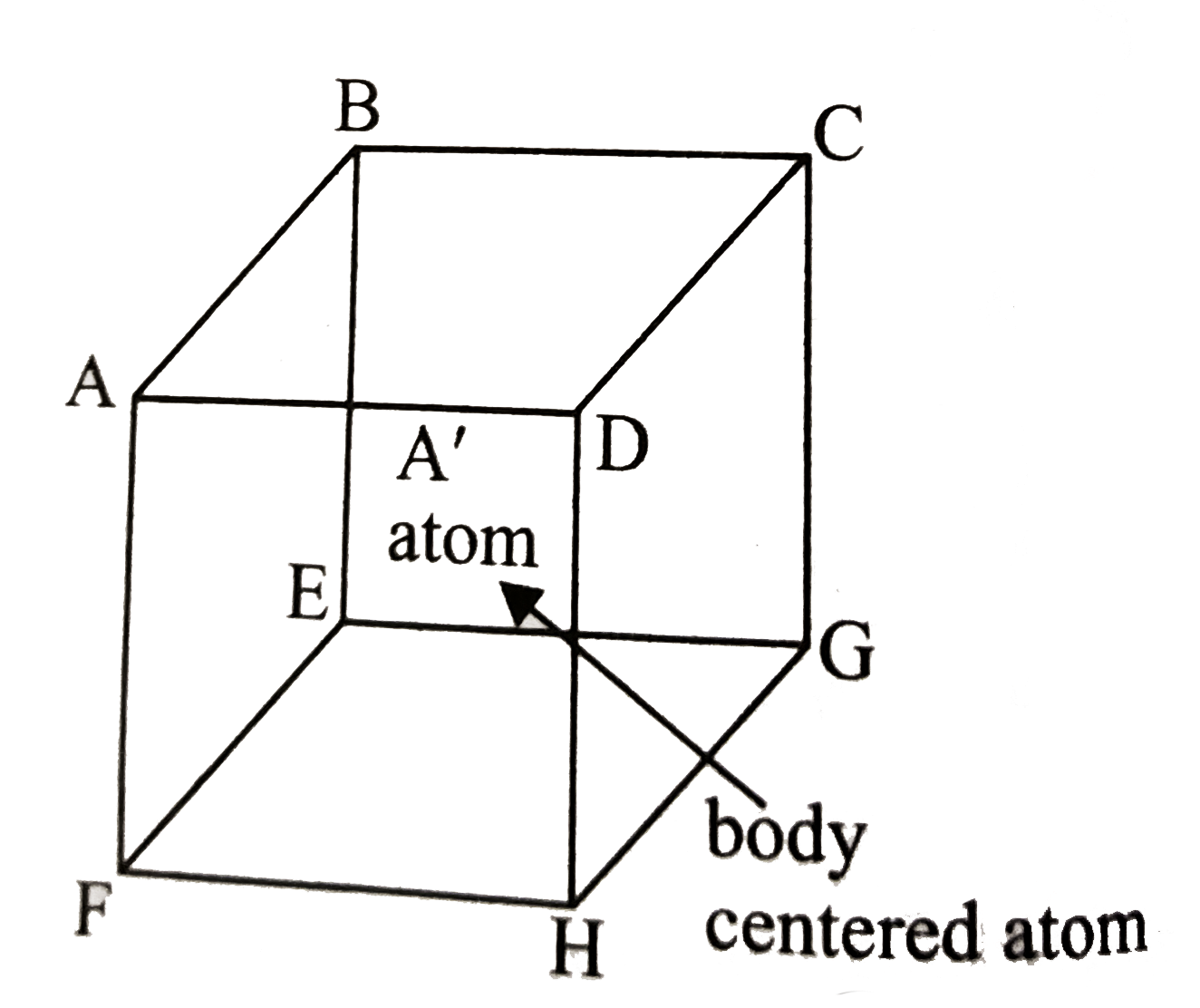
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
RESONANCE ENGLISH|Exercise Exercise -2 (Part-IV Comprehension)|8 VideosSOLID STATE
RESONANCE ENGLISH|Exercise Exercise -3 (Part-I)|16 VideosSOLID STATE
RESONANCE ENGLISH|Exercise Exercise -2 (Part-II Single correct double value integer type)|9 VideosS BLOCK ELEMENTS
RESONANCE ENGLISH|Exercise ORGANIC CHEMISTRY(Biomolecules & Polymer)|4 VideosSTRUCTURAL IDENTIFICATION & PRACTICAL ORGANIC CHEMISTRY
RESONANCE ENGLISH|Exercise Section -B|18 Videos
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-SOLID STATE-Exercise -2 (Part-III)
- Which of the following statements is/are false
Text Solution
|
- Amorphous solids can also be callled ………….. .
Text Solution
|
- In body-centred cubic lattice given below, the three disntances AB, AC...
Text Solution
|
- A metal crystallises in bcc. Find % fraction of edge length not covere...
Text Solution
|
- Select the correct statement(s) about three-dimensional hcp system.
Text Solution
|
- Which of the following is/are ture about HCP and CCP lattice?
Text Solution
|
- In which of the following arrangments octahedral voids are formed ?
Text Solution
|
- the number of tetrahedral voids per unit cell in NaCl crystal is ...
Text Solution
|
- Which of the following statements are correct
Text Solution
|
- Which of the following is/are correct ?
Text Solution
|
- A perfect crystal of silicon (Fig.) is doped with some elements as giv...
Text Solution
|
- Which of the following statements are true about semiconductors ?
Text Solution
|