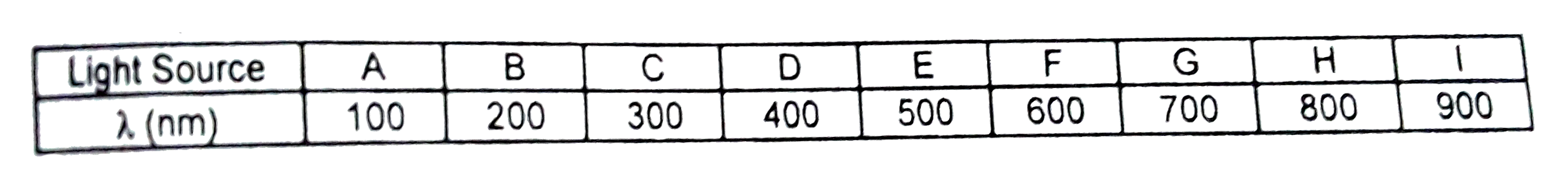
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-DPP-QUESTIONS
- Which of the following statements is correct?
Text Solution
|
- The energy required to remove an electron from metal X is E = 3.31 xx ...
Text Solution
|
- The wavelength (lambda) of monochromatic light coming from some light ...
Text Solution
|
- For which of the following species, Bohr theory doesn't apply
Text Solution
|
- If radius of second stationary orbit (in Bohr's atom) is R then radius...
Text Solution
|
- The radius of the first orbit of H-atom is r. then the radius of the f...
Text Solution
|
- The number of Br^- Ions in 2.1 g CaBr2 is [NA=6xx10^23]
Text Solution
|
- If the mass of 10^22 molecules of a hydrocarbon is about 1.2 g, then t...
Text Solution
|
- The value of R will be maximum for which of the following gases :
Text Solution
|
- Volume occupied by an ideal gas at one atmospheric pressure and 0^@C i...
Text Solution
|
- Which of the following exert highest pressure at 300 K and 4 L volume ...
Text Solution
|
- Match the List with the appropriate List Il and select the correct ans...
Text Solution
|
- Select the correct option
Text Solution
|
- 11.2 litre of a gas at STP weighs 14 g The gas could not be
Text Solution
|
- x g Urea (NH2CONH2) contains y total number of atoms 4x g Acetic acid ...
Text Solution
|
- Air in a room is separated in two regions in volume ratio of 4: 1 by a...
Text Solution
|
- Find the average molar mass of a gaseous mixture in g/mole, which cont...
Text Solution
|
- The triad of nuclei that is isotonic is
Text Solution
|
- "A^(+2)" is isoelectronic with N2O and has (Z+1) neutron (Z is atomic ...
Text Solution
|
- Select the correct statement
Text Solution
|