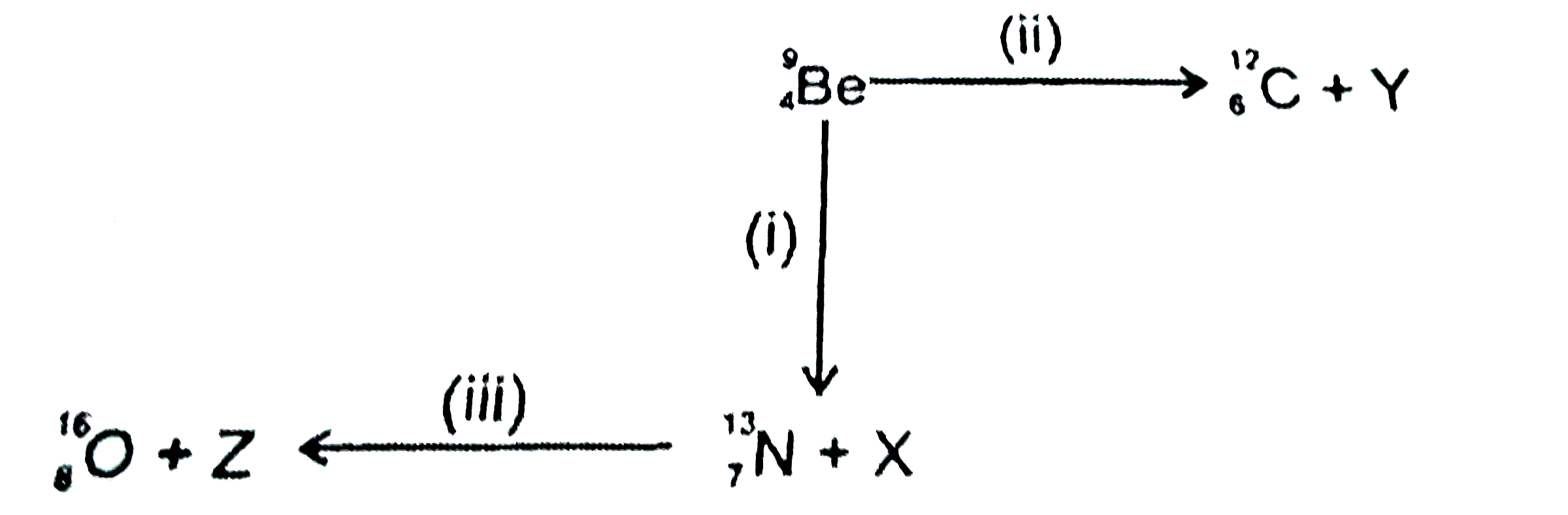A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE ENGLISH-DPP-QUESTIONS
- In a sample one H atom is in 1^(st) excited state, two He^+ ion are in...
Text Solution
|
- Among the following , which orbital in Be^(3+) has same energy as that...
Text Solution
|
- Bombardment by alpha-particle leads to artificial distintegration in t...
Text Solution
|
- Which particle must have been produced in the following processes resp...
Text Solution
|
- A neutral atom of an element has 2K, 8L, 9M and 2N electrons . Which o...
Text Solution
|
- 4.0 g of caustic soda (NaOH) contains same number of sodium ions as ar...
Text Solution
|
- If the binding energy of 2nd excited state of a hypothetical H-like at...
Text Solution
|
- if the radius of the first Bohr's orbit of H-atom is x which of the fo...
Text Solution
|
- d(z^(2))-orbital has:
Text Solution
|
- Which of the following process lead to formation of isobars ?
Text Solution
|
- In the disintegration of a radioactive element, alpha- and beta-partic...
Text Solution
|
- In the disintegration of a radioactive element, alpha- and beta-partic...
Text Solution
|
- In the disintegration of a radioactive element, alpha- and beta-partic...
Text Solution
|
- The de-Broglie wavelength of electron in a certain orbit 'n' of Be^"3+...
Text Solution
|
- Find the value of x+2y+z x=no. of radial nodes in 3px , y=no. of ang...
Text Solution
|
- .72Re^162 to.(z1)X^(A1)+ alpha particle .74W^(188)to.(Z2)Y^(A2)+Be...
Text Solution
|
- Which of the following is saturated hydrocarbon ?
Text Solution
|
- A : Carbon has maximum tendency of catenation among group 14 . R : C...
Text Solution
|
- Which of the following is the empirical formula of C6H6 ?
Text Solution
|
Text Solution
|